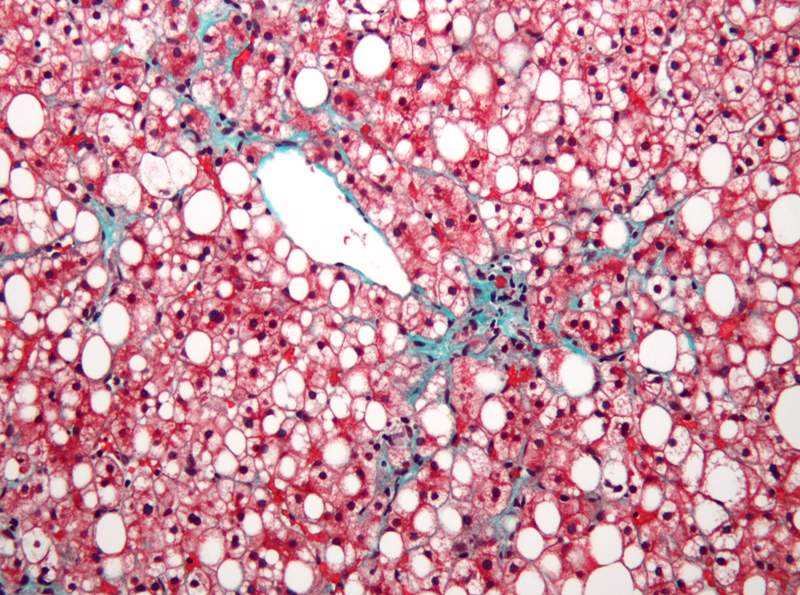
Gemphire Therapeutics has started a Phase lla proof-of-concept (PoC) clinical trial to evaluate gemcabene as a treatment for paediatric nonalcoholic fatty liver disease (NAFLD).
The open-label study aims to enrol around 40 adolescent children between the age groups of 12 and 17 who are diagnosed with NAFLD and abnormal liver function as assessed by liver transaminases.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
During the trial, patients will be given a 300mg dose of gemcabene once-daily.
Primary endpoint of the trial includes a measure of the change in serum alanine transaminase (ALT), an enzyme that serves as a biomarker of liver function, from baseline to 12 weeks.
Secondary endpoints feature change in hepatic steatosis as measured by non-invasive magnetic resonance imaging-proton density fat fraction (MRI-PDFF), change in liver inflammation and fibrosis (LIF) score by non-invasive MRI liver multiscan.
It also includes change in AST, insulin sensitivity, serum lipids (including triglycerides), apolipoproteins, and inflammatory markers (including hsCRP), as well as safety and tolerability.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTop-line results from the trial are expected by early next year.
Gemphireinterim president and CEOSteven Gullans said: “We believe gemcabene, based on its favourable safety profile and novel mechanism targeting the underlying pathologies of both dyslipidemia and inflammation, will have a distinct competitive advantage.
“Given the paucity of clinical trials in paediatric NAFLD, and the large number of adolescents already identified with this condition, we expect to recruit paediatric patients faster than is currently possible in similar adult trials.”
Furthermore, Gemphire seeks to conduct sub-studies to assess gemcabene’s pre and post-treatment effects on the duration of elevated fat in the blood after a meal (post-prandial lipemia), and measurement of the liver’s ability to synthesise triglycerides (de-novo lipogenesis), which are both forms of metabolism that is usually elevated in NAFLD patients.





