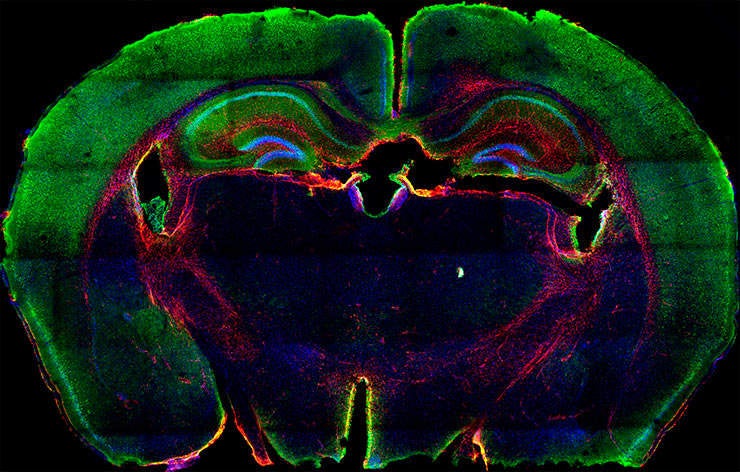
Researchers from the Seve Ballesteros Foundation-CNIO Brain Tumour Group at the Spanish National Cancer Research Centre (CNIO) have created a novel mouse model capable of accurately recreating genetic alterations found in patients with gliomas, a type of brain tumour that originates in the glial cells.
The model was made using the genome editing tool CRISPR-Cas9 in combination with gene delivery system RCAS/TVA. It is hoped that it will help accelerate pre-clinical testing of targeted therapies and improve cancer research through providing a versatile model for clinical testing.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
“A current high priority in cancer research is to functionally validate candidate genetic alterations that are relevant for cancer progression and treatment response,” the research team said.
“In order to do so, it is essential to develop flexible models that can speed up the identification of cancer driver genes among the large number of passenger alterations.”
The team, led by Massimo Squatrito, combined the two technologies to generate a mouse model capable of mimicking the genetic structure and complexity of a cancer tumour. Study co-authors Barbara Oldrini and Alvaro Curiel-Garcia used the new model to recapitulate some genetic alterations found in gliomas.
Particular focus was given to NTRK, a gene fusion encoding a family of kinases, as well as a common mutation of the BRAF gene. Both are present in tumour types other than glioma.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“What we have shown using this new model is that we now have the ability to generate specific complex genetic alterations and to study how they contribute to glioma pathogenesis”, Squatrito said.
The research team also used the new model to examine different therapeutic approaches currently used in clinics, as well as analyse the mechanisms of resistance which could cause tumour recurrence.
The findings helped the team to identify possible treatment alternatives which could be used to overcome the acquired resistance to TRK and BRAF inhibitors.
“We can efficiently recreate a variety of genetic alterations, including gene translocations and point mutations, and we can move fast from the mouse model to the translational studies,” Squatrito said.
“Here we have shown that this approach is feasible and we believe that such a flexible model will greatly accelerate the pre-clinical testing of novel targeted therapies.”
The findings were published in the journal Nature Communications.





