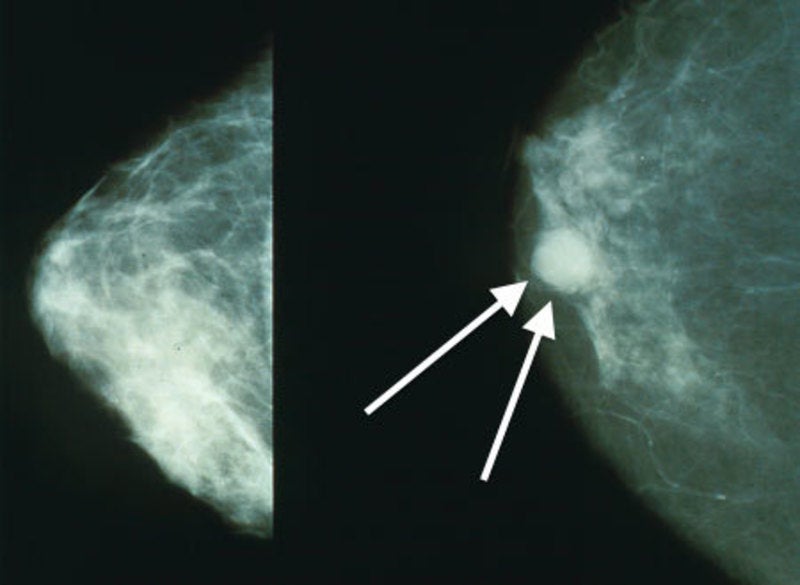
Generex Biotechnology will manage a Phase II clinical trial of pembrolizumab (keytruda) for the treatment of patients with metastatic triple negative breast cancer after signing an agreement with the NSABP Foundation.
Keytruda will be trialed in combination with the AE37 peptide vaccine under the clinical trial agreement (CTA).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
NSABP will provide clinical trial and site management services for Generex’s wholly-owned subsidiary Antigen Express and research partner Merck.
Antigen Express president Eric von Hofe said: “We are very pleased to be working with the NSABP Foundation on this important trial combining AE37 and keytruda in triple-negative breast cancer patients.
“The extensive expertise of the NSABP Foundation and their network of sites and investigators will be a great asset in this development effort.”
Sponsored by Generex, the trial is being conducted in conjunction with Merck and is currently being reviewed by the US Food and Drug Administration (FDA).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataClinical operations including site qualification, drug shipment and packaging, and IRB review and approval are currently underway.
Generex R&D executive vice-president Richard Purcell said: “This contract with our research partners at the NSABP Foundation provides cost and timeline certainty to our AE37 development programme in combination with keytruda.”
The company had earlier filed an investigational new drug application with the FDA to initiate the Phase II clinical trial of keytruda in combination with AE37 therapeutic cancer vaccine.
AE37 vaccine is a combination of portions of two proteins that together stimulate the immune system to fight cancer cells. It is currently being developed to treat certain types of breast cancer in women.
The first patients for the trial are expected to be enrolled in the first quarter of next year.





