Visit our Covid-19 microsite for the latest coronavirus news, analysis and updates
Follow the latest Covid-19 updates on our timeline.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Amid the ongoing Covid-19 pandemic, Genfit is monitoring the impact on regulatory and clinical activities associated with its existing programmes.
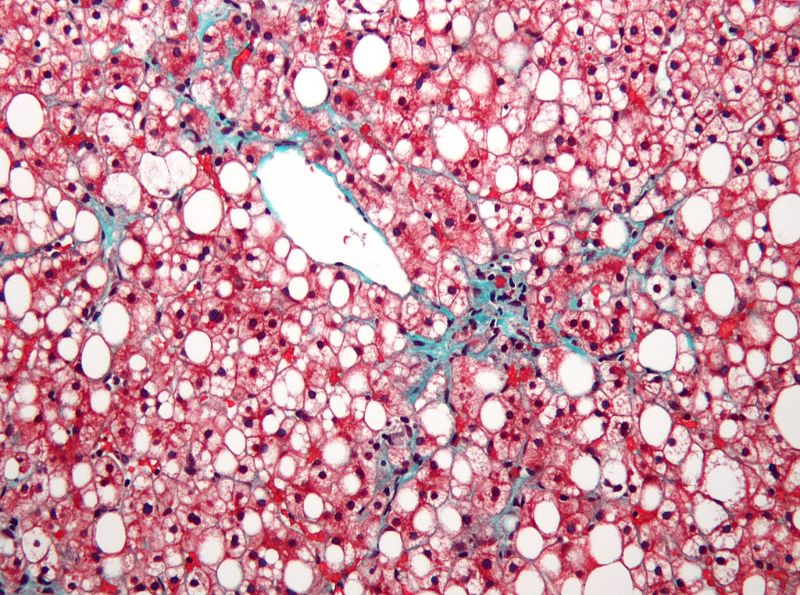
The company does not expect any significant delay in the interim data readout of its Phase III RESOLVE-IT clinical trial of elafibranor involving patients suffering from non-alcoholic steatohepatitis with fibrosis.
According to Genfit, around 1,000 initial participants needed for regulatory approval have undergone their final visits. The cohort’s database was locked in February.
The unblinding of the study and interim data reporting will be based on the receipt and integration of insight from the US Food and Drug Administration (FDA).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGenfit also decided to continue the extension phase of RESOLVE-IT trial. The company adopted certain safety measures for patients, including virtual clinic visits and home delivery of study drug.
However, the screening of new participants has been temporarily suspended.
The company placed on hold all Phase I trials, including pharmacokinetic, food effect and bioequivalence studies. Genfit said that these trials are required for filing elafibranor’s NDA in NASH indication.
Other trials on hold are the pharmacokinetic/pharmacodynamic paediatric NASH study and the Phase II liver fat study.
The company’s NASH combination and PBC programmes are being continued, and the launch of Phase II combination trial and Phase III PBC trial are on hold.
The company said in a statement: “All supporting activities pertaining to continuation of ongoing studies or the initiation of new studies will continue in order to minimise potential delays when the pandemic crisis subsides.”
The company is not currently expecting any supply disruption for studies but will continue to monitor the situation. It has enough elafibranor supply for all studies up to mid-2021.





