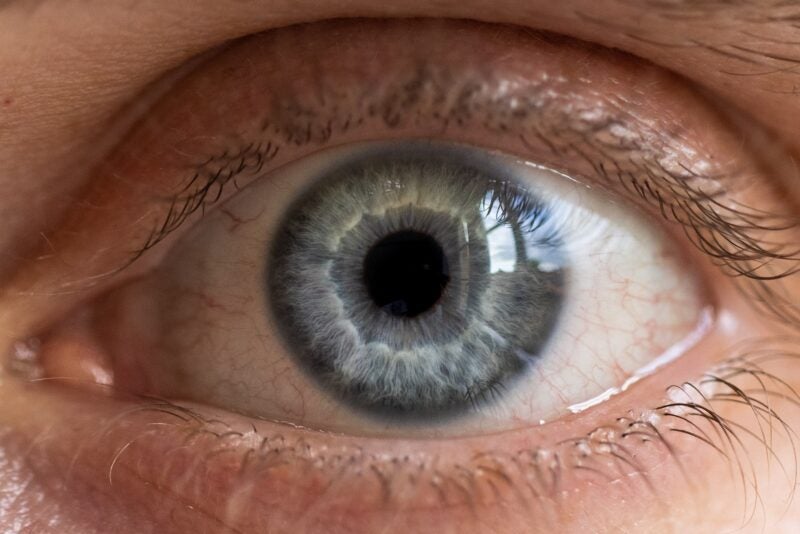
Glaukos has announced the first subject enrolment in the Phase II clinical trial of its investigational drug candidate GLK-301 to treat dry eye disease (DED) signs and symptoms.
Pilocarpine is the active pharmaceutical ingredient of GLK-301, which uses the cream-based drug formulations of Glaukos’ iLution platform.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Applied to the exterior surface of the eyelid, these formulations are intended for dropless transdermal pharmaceutically active compound delivery to treat eye ailments.
The sterile ophthalmic topical cream formulation serves as a depot permitting delivery of pilocarpine to the eye via the eyelid dermis.
The placebo-controlled, randomised, multicentre, double-masked trial will assess the safety and efficacy of three dose levels of GLK-301 given two times a day to the eyelids in DED patients versus placebo for 28 days.
Trial subjects will be followed up for another 14 days.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataStandard DED signs and symptoms will be the endpoints of the trial.
The company will enrol nearly 200 DED patients in the study across clinical sites in the US.
Additionally, approximately 20 patients with a DED diagnosis due to Sjogren’s Syndrome will be enrolled.
Glaukos president and CEO Thomas Burns said: “Dry eye disease is a common ocular inflammation condition with high unmet clinical need, representing one of the world’s largest ophthalmic markets worldwide.
“We believe our iLution platform has the potential to address the major unmet need for patients suffering from dry eye disease and other chronic eye diseases by providing an effective, easy to administer, safe, dropless transdermal therapeutic.”
Separately, Glaukos enrolled the first participant in a Phase II trial of GLK-302 to treat presbyopia.
The second investigational drug candidate, GLK-302, uses iLution platform’s cream-based drug formulations.





