GRAIL is ensuring its multi-cancer early detection test will be evaluated amongst under-represented and underserved communities, as the company initiates its REACH study amongst Medicare beneficiaries.
The study will compare individuals who have an annual Galleri blood test plus usual care compared to those who receive usual care alone. GRAIL aims to enrol 50,000 Medicare beneficiaries.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The population cohort will include under-represented populations across race, ethnicity, socioeconomic status, and underserved communities.
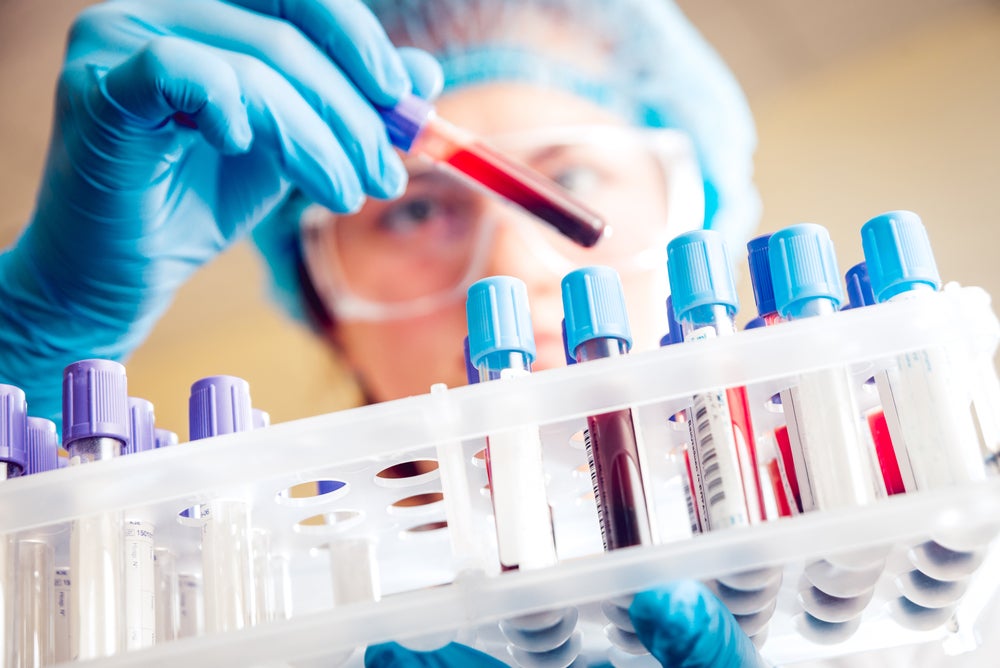
The Galleri test is investigational device exemption (IDE) approved and has already been tested in a multi-centre US study called PATHFINDER and an NHS-partnered trial in England.
Galleri is a blood-based multi-cancer early detection test that can detect abnormal DNA produced by more than 50 types of cancer. It can predict the location or origin of the cancer once processed.
GRAIL said that the Medicare study will reveal impact measures such as the tests’ ability to reduce diagnosed stage IV cancers and how healthcare resources are used in the cohort that has the annual tests.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe interim data will be available for the Centers for Medicare and Medicaid to look over in the event of a premarket approval application (PMA) approval by the US Food and Drug Administration.
The results will add to a wealth of data already produced by GRAIL. Results from the PATHFINDER study were published in The Lancet – demonstrating that screening with the test resulted in a significantly greater amount of cancer detection compared to normal screening methods.
The NHS-partnered trial is currently ongoing with more than 140,000 participants.
Around two-thirds of the deaths from cancer in the US occurred in people aged 65 years and older.
GRAIL CEO Bob Ragusa said: “Multi-cancer early detection holds the promise to detect more cancers earlier, improve cancer outcomes, and reduce overall cancer costs, but only if accessible to all seniors.”
“The Galleri-Medicare study demonstrates our commitment to provide broad, equitable access to early cancer detection that is representative of the US population, including groups that are often under-represented in clinical research.”



