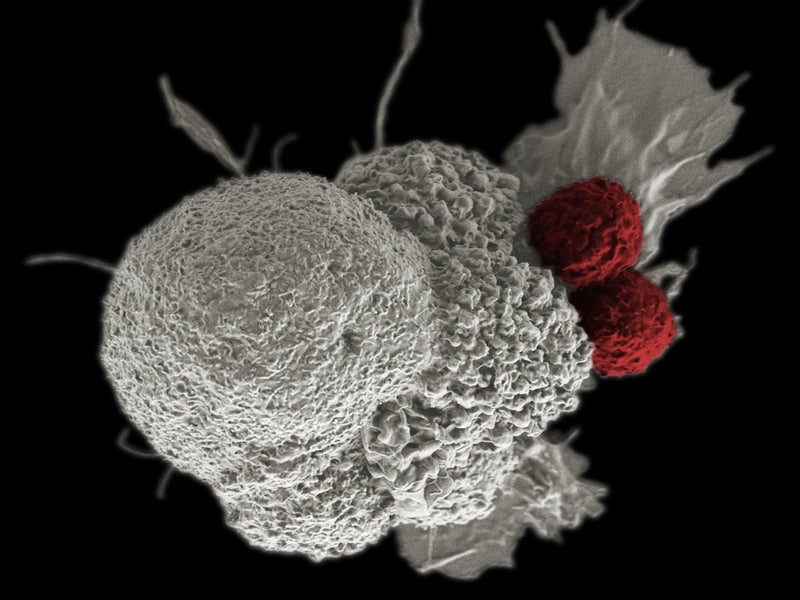
Gritstone Oncology has commenced dosing of patients in a Phase I/II clinical trial evaluating its personalised immunotherapy candidate GRANITE-001 in advanced solid tumours.
GRANITE-001 is designed to target and trigger significant T-cell response against the patient’s tumour-specific neoantigens, which will be detected using Gritstone’s EDGE artificial intelligence platform.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The therapeutic comprises a priming adenoviral vector and a monthly boosting with an RNA vector. Both vectors contain the same 20 patient-specific tumour-specific neoantigens.
Known as GO-004, the Phase I/II trial will assess GRANITE-001 plus immune checkpoint blockade to treat advanced solid tumours, including microsatellite stable colorectal cancer, gastroesophageal cancer, metastatic non-small cell lung cancer, and bladder cancer.
Gritstone Oncology co-founder, president, and CEO Andrew Allen said: “With GRANITE-001, we are analysing the patient’s own tumour cell data through our artificial intelligence platform EDGE and using the identified neoantigens as the basis of a potent, virus-driven, personalised immunotherapy candidate which we manufacture in-house in large part.
“Our team has impeccably executed the design, build-out, and implementation of this unique platform and our clinical trial collaborators have joined us in this exciting and innovative effort to apply novel scientific insights to the treatment of this grim disease.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEnrolment is ongoing for the trial’s Phase I portion, which involves two parts.
During part A, subjects will be administered with a full, fixed dose of adenovirus-based prime vector with escalating doses of RNA-based boost vector in combination with an intravenous anti-PD-1 therapy called nivolumab.
The part B will evaluate the prime and boost vectors at the selected dose plus nivolumab and also subcutaneous anti-CTLA-4 antibody ipilimumab.
Preliminary results from the trial are expected to be reported in the fourth quarter of this year.
GRANITE-001 has the US Food and Drug Administration (FDA) fast track designation for the treatment of MSS CRC.





