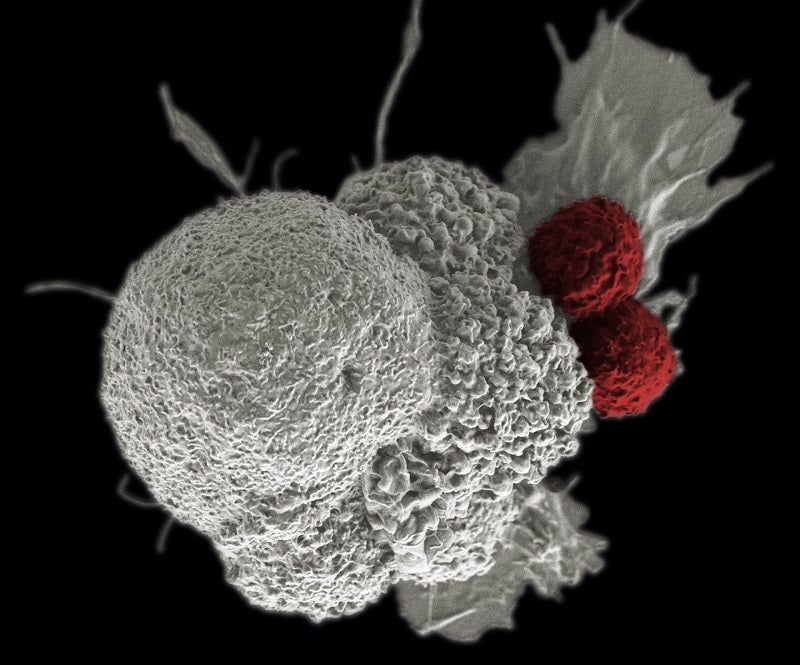
HiFiBiO Therapeutics has announced a clinical trial supply agreement with Novartis to assess its HFB200603, along with the latter’s tislelizumab, to treat advanced solid tumours.
The latest deal represents the second clinical trial supply agreement with Novartis, following the first agreement announced in August this year.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Under the agreement terms, Novartis will be responsible for supplying tislelizumab, and HiFiBiO Therapeutics will maintain control of the HFB200603 programme, along with the global research and development and commercial rights.
HiFiBiO Therapeutics will assess the combination of its new anti-BLTA monoclonal antibody, HFB200603, and tislelizumab to treat advanced solid tumour indications preselected by the company’s Drug Intelligence Science (DIS) platform.
HiFiBiO Therapeutics founder, chairperson, and CEO Liang Schweizer said: “The continuation of our collaboration with Novartis bring us a step closer to realise our vision to provide potentially curative immunotherapies for each and every patient using our Drug Intelligence Science (DISTM) approach for novel drug discovery and development.”
The company’s single cell platform has identified the monoclonal antibody as a single digit nanomolar binder to human and cynomolgus BTLA.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHFB200603 also has the capability to block the BTLA interaction with its ligand HVEM and reverse the HVEM-mediated immune suppressive effects.
It can also induce the inflammatory cytokines production in the tumour microenvironment of different human tumour types.
Novartis’s humanised IgG4 anti-PD-1 monoclonal antibody tislelizumab has been specifically designed for reducing binding to Fc-gamma (Fcγ) receptors on macrophages.
In 2020, HiFiBiO Therapeutics concluded the first cohort of the Phase I trial of HFB30132A, a SARS-CoV-2 neutralising antibody, for the treatment of Covid-19.





