Horizon Pharma has enrolled the first patient in a Phase ll MIRROR clinical trial investigating Krystexxa (pegloticase injection) in combination with methotrexate for the treatment of adults living with uncontrolled gout.
The trial intends to study the efficacy, safety, tolerability, and blood levels of concomitant use of Krystexxa and methotrexate, administered to prevent immunogenicity against Krystexxa, in patients with uncontrolled gout.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Around 30 patients are expected to be enrolled in the multi-centre, open-label, single-group assignment trial.
During the trial, the patients will receive a 15mg once-weekly oral dose of methotrexate, starting four weeks before and continued through to 24 weeks of bi-weekly infusions of Krystexxa.
The trial’s primary endpoint is proportion of serum uric acid responders (sUA) at month three.
Its secondary endpoints are proportion of sUA at month six, proportion of (sUA) in month three and month six combined, mean change from baseline in serum uric acid from baseline through to week 24, among others.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataHorizon Pharma vice president and rheumatology therapeutic area head Paul Peloso said: “Real-world feedback from the medical community informed our selection of methotrexate as the immunomodulator in this study.
“MIRROR complements two ongoing investigator-initiated trials of Krystexxa with other commonly used immunomodulators, azathioprine and mycophenolate mofetil.
“This trial is part of our comprehensive clinical strategy to address the burden of uncontrolled gout.”
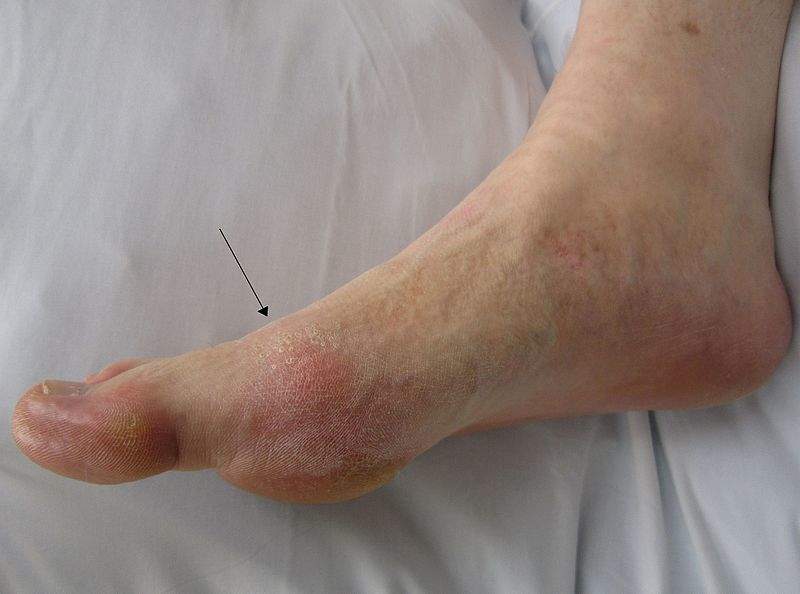
Gout is a chronic, progressive inflammatory form of arthritis which is a result of excessive formation of uric acid in the body and needs aggressive management.
Patients suffering from the condition continue to have abnormally high levels of uric acid along with symptoms of gout even after using conventional therapies.





