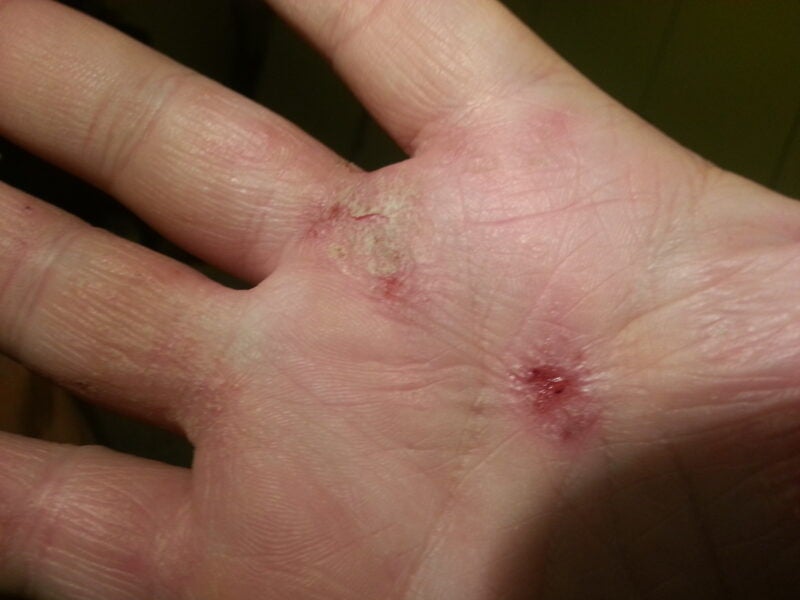
Hoth Therapeutics has reported positive findings from a Phase Ib clinical trial of its investigational therapy, BioLexa, in adult mild-to-moderate atopic dermatitis patients.
According to the findings, the trial met primary and secondary endpoints of safety and efficacy.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
All subjects demonstrated improvements during the trial versus the day one score on using the SCORing Atopic Dermatitis (SCORAD) as a clinical tool for evaluating atopic dermatitis severity and Eczema Area and Severity Index (EASI) score.
The EASI score is utilised to evaluate atopic eczema extent (area) and severity.
During the complete 28-day trial duration, all subjects showed a clinically relevant improvement of >50% on the EASI scale.
Nearly 60% of the subjects had an overall clinically relevant improvement by SCORAD during the trial versus the day one score.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn addition, 42% of participants had preserved improvement from day 14 to 28, which comprises two weeks without active therapy.
This backs the long-term effect of BioLexa based on the mechanism of action for treating the underlying staphylococcal infection.
In the trial, 71% of subjects who received BioLexa had a clinically relevant decline in the atopic dermatitis-affected total body surface area.
BioLexa was found to be well tolerated without any serious adverse events and drug-associated treatment-emergent adverse events.
Hoth Therapeutics CEO Robb Knie said: “The positive results give us even more confidence that BioLexa can help provide relief from atopic dermatitis in patients, without the harmful long-term effects of corticosteroids and other side effects that many patients suffer with today’s choice of approved therapeutics.”
In June 2019, the company announced the formation of its subsidiary, Hoth Therapeutics Australia, in preparation for clinical studies.





