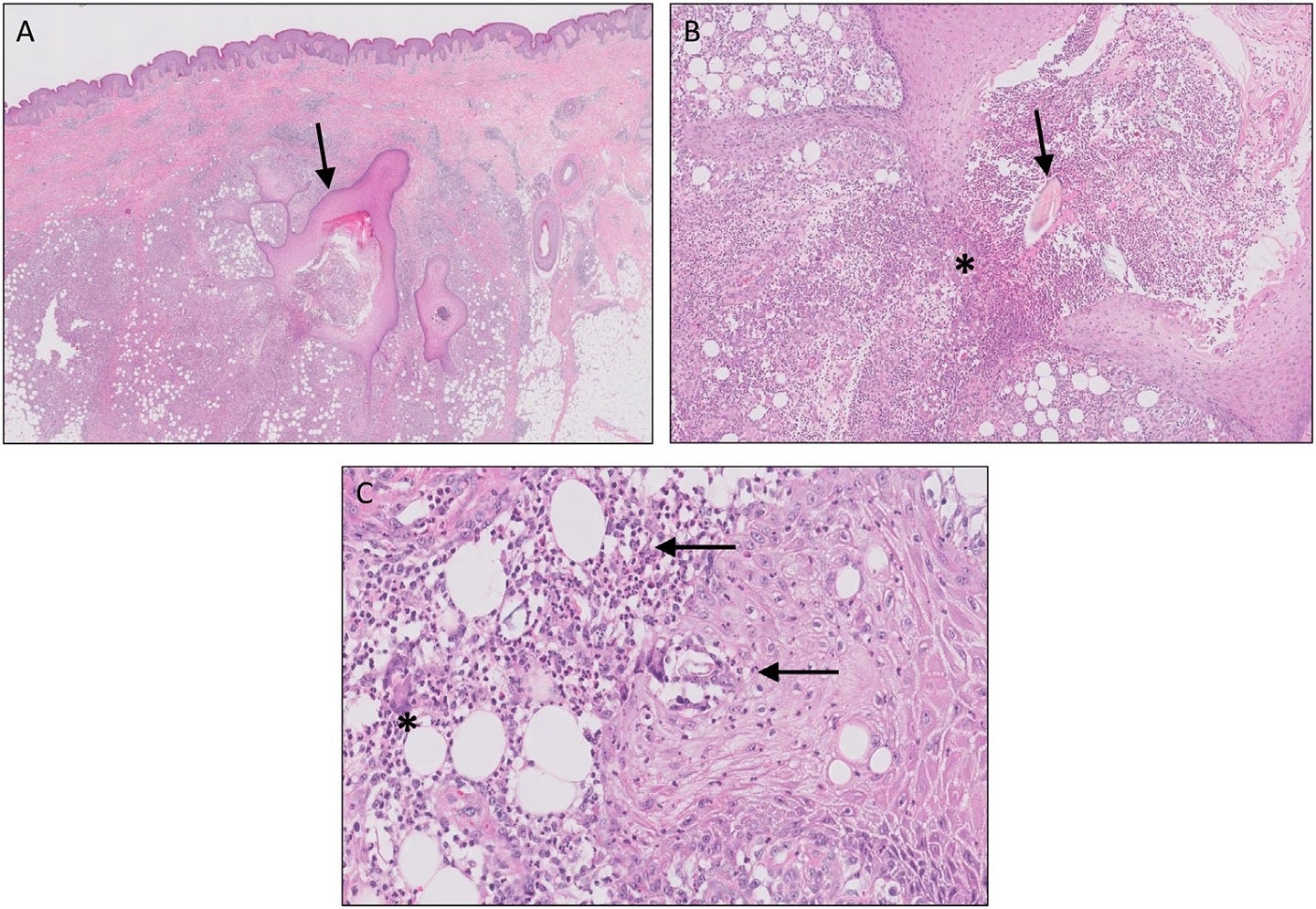
Incyte has reported new 52-week data from a Phase II trial of oral JAK1 inhibitor, povorcitinib (formerly INCB54707), in adult hidradenitis suppurativa (HS) patients.
The double-blind, dose-ranging, randomised, placebo-controlled Phase II trial was designed for assessing the safety and efficacy of povorcitinib in these patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Findings showed that longer-term treatment with povorcitinib 75mg once daily (QD) resulted in sustained and durable efficacy across all treatment arms.
They include the 36-week open-label extension period during which all participants who have received povorcitinib 75mg QD demonstrated sustained efficacy for all treatment arms after switching to this dose.
At week-52, the oral inhibitor also showed durable efficacy in high-threshold outcomes.
It was also found to be generally well tolerated and showed a safety profile that is consistent with the previous data.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIncyte Drug Development, Inflammation & AutoImmunity associate vice-president and Povorcitinib global programme head Kurt Brown said: “HS is a chronic, progressive and debilitating condition for which there is no cure.
“We are encouraged by these Phase II data and believe they reinforce the potential of povorcitinib to be a safe and efficacious treatment for HS that is tolerable with longer-term administration, even at the higher doses.
“Despite the available treatments for HS, no uniformly effective therapy has been found, underscoring the need for additional options. We look forward to continuing to progress the development of povorcitinib through our ongoing Phase III trial in patients with moderate-to-severe HS.”
Upper respiratory tract infection, urinary tract infection, nasopharyngitis, increased blood creatine kinase (CK), Covid-19, headache, and acne were the most common treatment-emergent adverse events observed at week-52.





