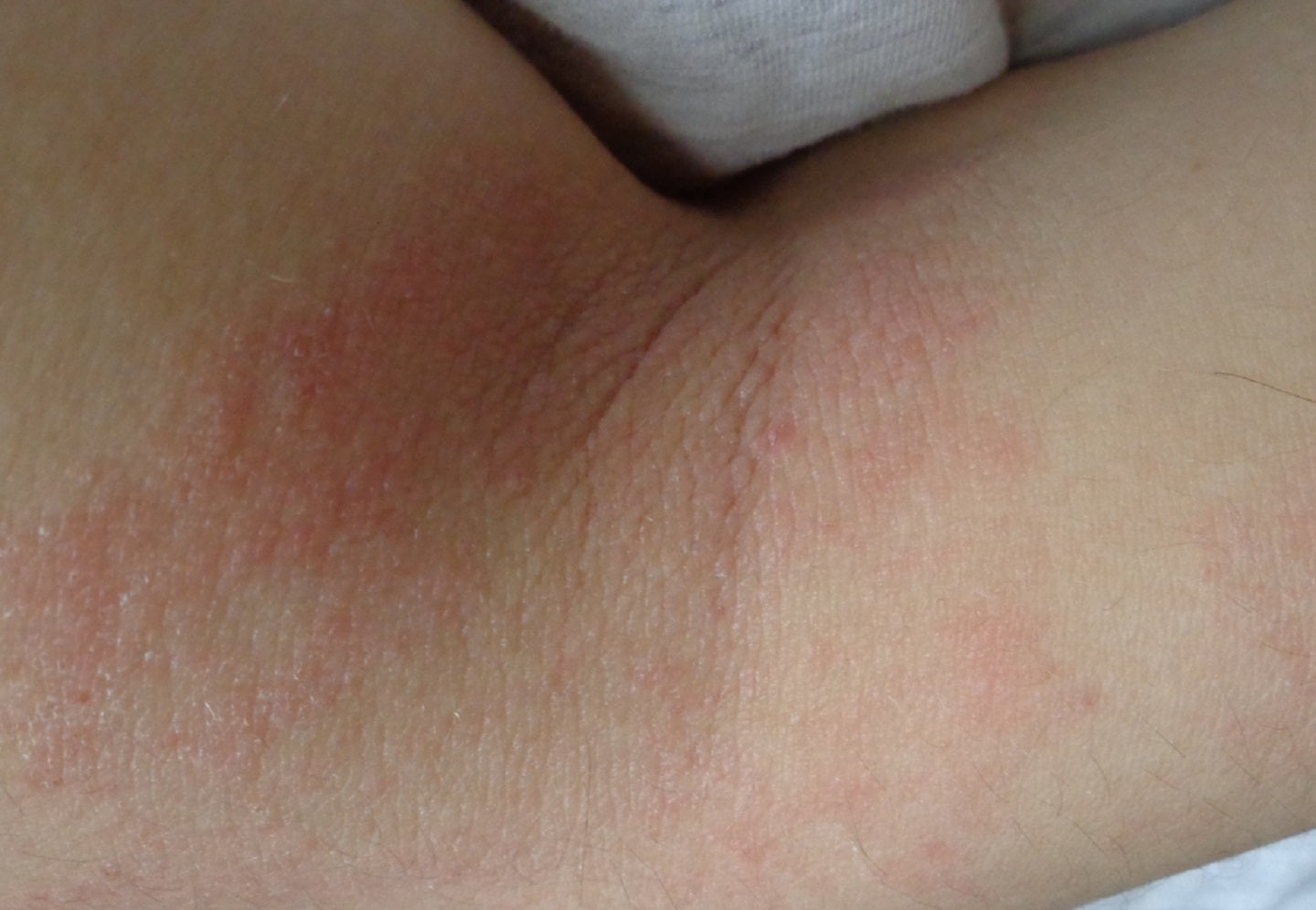
Incyte has announced results from its Phase II SCRATCH-AD trial assessing the short-term clinical benefits of Opzelura (ruxolitinib) cream 1.5% to control pruritus and reduce disease severity in adult atopic dermatitis (AD) patients.
The single-site, open-label, single-arm trial enrolled 49 adult patients aged 18 to 65 years who were diagnosed with AD for ≥6 months and with chronic itch related to AD for ≥3 months.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has been designed to assess the efficacy and safety of the cream in these patients.
For 28 days, patients applied Opzelura 1.5% cream two times a day approximately 12 hours apart to all lesions that were identified at baseline and any new lesions.
AD treated patients with Opzelura reported a rapid and substantial improvement in itch, which was sustained through day 28.
The study also met its primary endpoint, a change from baseline in peak pruritus numerical rating scale (PP-NRS) on day 2 (24 hours after the first application of Opzelura).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn addition, Opzelura was found to be well tolerated and no serious treatment-emergent adverse events were observed during the study period of 28 days.
Incyte Inflammation and AutoImmunity group vice-president Jim Lee said: “AD is a chronic, immune-mediated skin condition that can be difficult to manage and causes disruptive and persistent symptoms like itch that can greatly affect patients’ everyday lives.
“We are pleased that the SCRATCH-AD results further emphasise the rapid impact of Opzelura on itch reduction and reinforce its profile as an effective, well-tolerated topical non-steroidal treatment for AD.”





