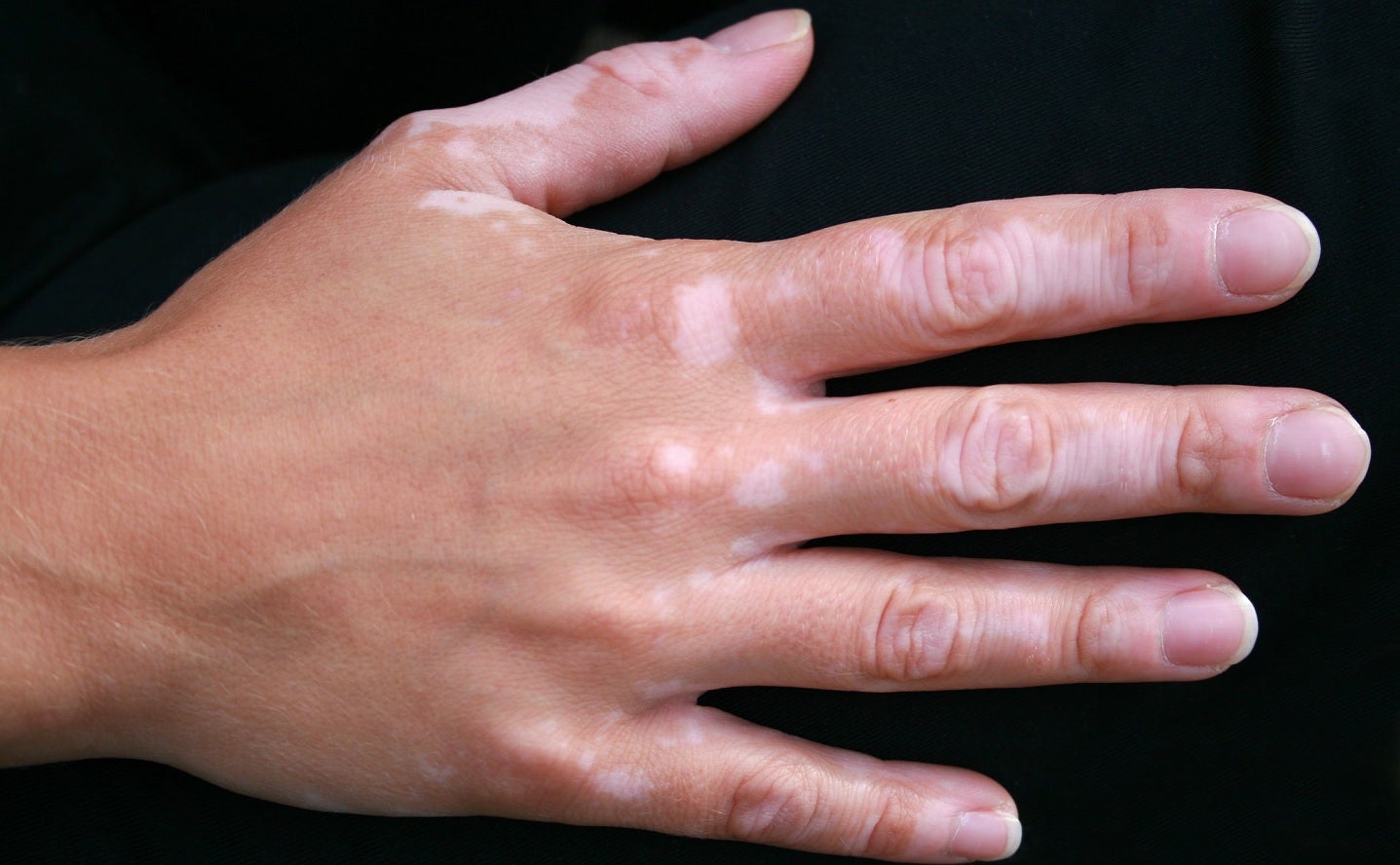
Incyte has reported data from a Phase IIb clinical trial of povorcitinib (INCB54707) in extensive nonsegmental vitiligo adult patients.
The placebo-controlled, randomised, dose ranging, double-blind Phase IIb trial has been designed for assessing povorcitinib’s safety and efficacy in these patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
In the trial, 171 participants aged 18 to 75 years, who are diagnosed with nonsegmental vitiligo affecting ≥8% of their body surface area, were enrolled and randomised into 1:1:1:1 ratio to receive 15mg, 45mg, 75mg povorcitinib once-daily (QD) or placebo for 24 weeks.
According to the findings, participants treated with povorcitinib had improvements in total body and facial repigmentation.
They also had statistically superior improvements in total Vitiligo Area Scoring Index (T-VASI) compared to placebo at week 24, and the trial also met its primary endpoint.
In addition, patients treated with povorcitinib achieved ≥50% reduction from baseline in T-VASI, the trial’s key secondary endpoint, at week 24.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPovorcitinib was also found to be well tolerated, with no serious treatment-emergent adverse events (TEAEs) observed in the trial.
Incyte Drug Development, Inflammation & Autoimmunity associate vice-president, and Povorcitinib global programme head Kurt Brown said: “Vitiligo is a chronic, immune-mediated disease which, until recently, had limited treatment options available to patients.
“We are proud to have brought to market the first and only US Food and Drug Administration (FDA) approved pharmacologic therapy for vitiligo, and continue to develop additional treatments for patients with vitiligo.
“These data suggest the potential of povorcitinib as an oral treatment for patients with extensive nonsegmental vitiligo and its potential versatility across multiple autoimmune and inflammatory conditions, including hidradenitis suppurativa for which we recently announced 52-week Phase II results.”
Headache, blood creatine phosphokinase increase, Covid-19, acne, and fatigue were the most common TEAEs observed during the 24-week placebo-controlled period.





