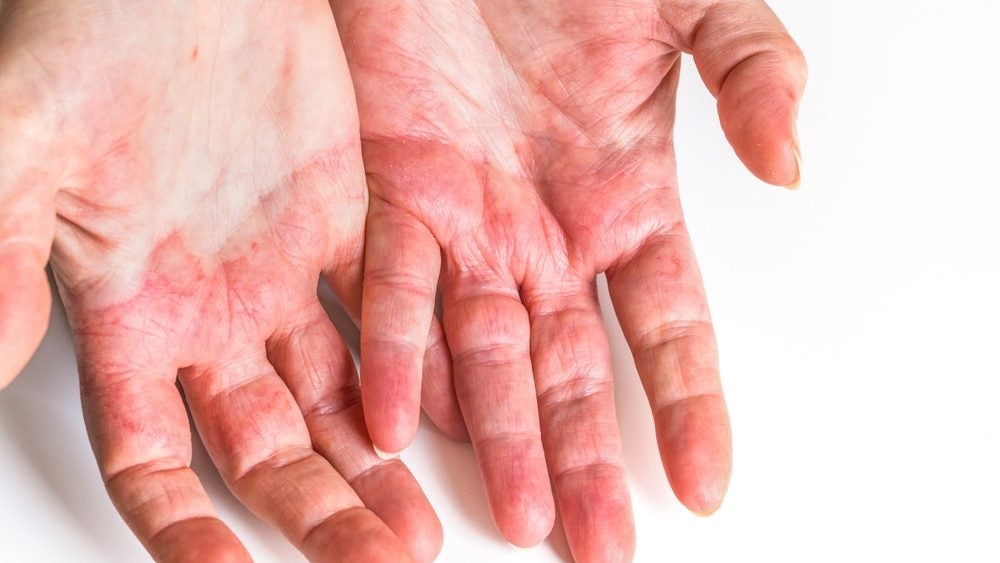
China-based biopharmaceutical company InnoCare Pharma has reported positive topline results from the Phase II clinical trial of ICP-332 to treat atopic dermatitis.
The randomised, double-blind, placebo-controlled Phase II trial (NCT05702268) investigated the safety, efficacy, and pharmacokinetics of ICP-332 in 75 patients with moderate-to-severe atopic dermatitis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The once-daily dosing of 80mg and 120mg demonstrated significant efficacy, with a mean percentage change from baseline in the Eczema Area and Severity Index (EASI) score reaching 78.2% and 72.5%, respectively, compared to 16.7% for the placebo group.
The findings suggest a favourable safety profile and substantial improvement in AD symptoms for patients in the study.
Other endpoints included adverse events, systolic and diastolic blood pressure, pulse rate, and electrocardiogram (ECG) QT interval, all up to 24 weeks. Additionally, the trial measured the number of participants with treatment-related adverse events as assessed by CTCAE v4.0.
ICP-332 is a tyrosine kinase 2 (TYK2) inhibitor. TYK2 is an enzyme involved in inflammatory signalling pathways related to the immune system. Inhibiting TYK2 can modulate inflammatory signals, potentially reducing excessive immune activity.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn the announcement accompanying the results, InnoCare CEO Dr Jasmine Cui said: “The AD has huge unmet medical needs, and we are excited to see the positive results from the phase II study of ICP-332. We will further accelerate the clinical development to benefit patients with AD and other autoimmune diseases.”
According to a report on GlobalData’s Pharma Intelligence Center, global sales in the atopic dermatitis market are expected to grow to $16.7bn by 2030.
GlobalData is the parent company of Clinical Trials Arena.
InnoCare has several drugs in the pipeline, including orelabrutinib, which is a BTK inhibitor, a TYK2-JH2 inhibitor ICP-488, and ICP-189.
The first patient was dosed in a Phase II clinical trial investigating ICP-488 to treat moderate-to-severe psoriasis in July. ICP-488 demonstrated a well-tolerated safety profile in a Phase I clinical trial, which involved the administration of single and multiple ascending doses to psoriasis patients.
In July, InnoCare teamed up with ArriVent to investigate the combination therapy of ICP-189 and furmonertinib for the treatment of advanced non-small cell lung cancer (NSCLC).
InnoCare also obtained approval in China to commence a clinical trial of ICP-723 (zurletrectinib) for the treatment of paediatric subjects aged two to 12 years old in the same month. The asset is being developed for the treatment of advanced or metastatic solid tumours that harbour NTRK fusion genes.





