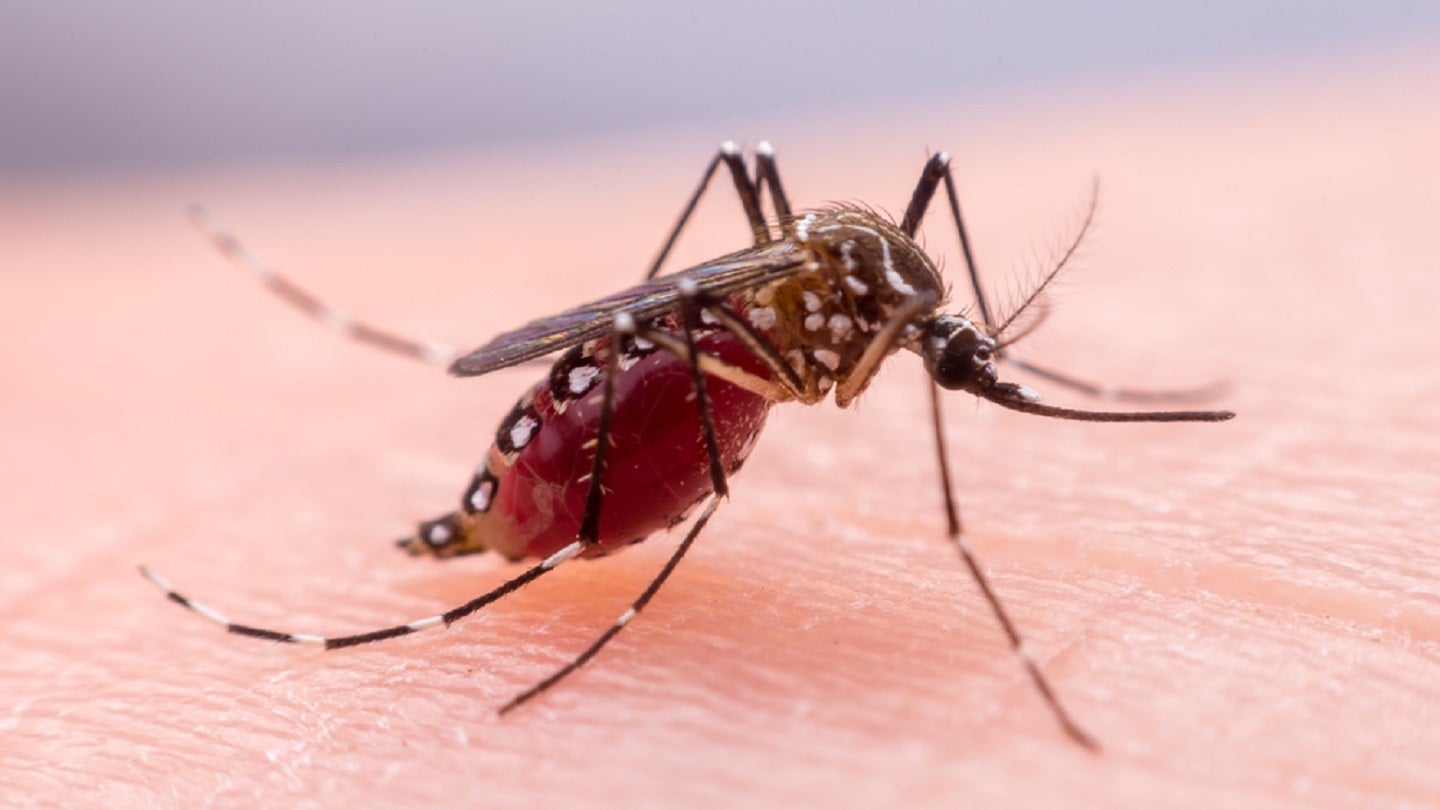
Island Pharmaceuticals is set to initiate a single ascending dose study of ISLA-101 drug for the prevention and treatment of dengue and other mosquito-borne diseases.
With the preparatory work mostly completed, the dose escalation study is expected to be initiated next month.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trial will be carried out in Australia with four cohorts of healthy subjects, who will receive escalating doses of ISLA-101.
Its objective is to make sure that the administered doses can safely reach the blood and act effectively against the dengue virus.
Under fasted conditions, subjects will receive increasing doses of ISLA-101 with the cohort receiving the highest dose that is safe, repeating it under fed conditions.
A safety review committee will review the safety data, after each cohort, and determine if it is safe to move to the higher dose.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIsland CEO and managing director Dr David Foster said: “We have been focused on moving the single ascending dose study along as quickly as possible and are very pleased to be nearing the commencement point.
“Putting this agreement in place with Beyond as our [contract research organisation] and naming Scientia as our clinical site are key to underpinning the study, with both organisations highly experienced in conducting studies like ours.”
Beyond Drug Development will conduct the trial and engage Scientia Clinical Research.
The engagement will involve recruitment of subjects, clinical site operations including housing of subjects, data management, statistical analysis, and project management.
Dosing will be completed late this year while the study readout is expected early next year.
Island plans to use the data from this study to further initiate the planned Phase IIa PEACH2 human clinical dengue challenge clinical trial.
The company can also make use of the Research and Development Tax Incentive scheme, which is offering rebates of up to 43.5% to compensate for each dollar spent on research and development activities.





