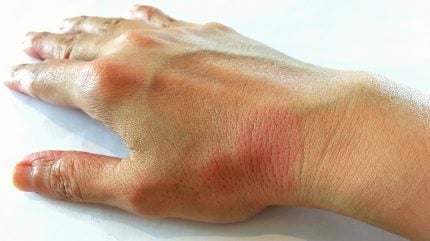
Jasper Therapeutics has dosed the first subject in its Phase Ib/IIa SPOTLIGHT clinical study of briquilimab in chronic inducible urticaria (CIndU).
Briquilimab is an antibody therapy targeting c-Kit (CD117) in mast cell-driven diseases such as CIndU and chronic spontaneous urticaria (CSU).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The SPOTLIGHT study’s primary endpoints are to assess the safety and tolerability of briquilimab in approximately 15 patients across two dose cohorts.
Secondary endpoints will evaluate the efficacy and pharmacokinetics of the treatment.
The clinical trial is being conducted across four European Union sites, with preliminary data expected in the latter half of this year.
Previously known as JSP191, briquilimab is an aglycosylated monoclonal antibody designed to block stem cell factors from attaching to c-Kit.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThis action disrupts survival signals, leading to the depletion of mast cells and potentially alleviating symptoms of mast cell-driven diseases, such as chronic urticaria.
Clinical studies of briquilimab are ongoing for patients with CSU, lower-risk myelodysplastic syndromes (LR-MDS), and as a conditioning agent for cell therapies targeting rare diseases.
Jasper chief medical officer Edwin Tucker said: “We are excited to announce the dosing of the first patient in the SPOTLIGHT study in patients with CIndU, our second clinical program evaluating briquilimab in a mast cell-mediated disease.
“As with our BEACON study in CSU, we expect the SPOTLIGHT study to establish proof of concept for the depletion of mast cells by briquilimab in CIndU and help us to determine doses and dosing regimens for future registrational studies.”
With more than 145 participants and healthy volunteers dosed so far, briquilimab has shown promise in various conditions, including severe combined immunodeficiency (SCID), acute myeloid leukaemia (AML), myelodysplastic syndromes (MDS), Fanconi anaemia (FA), and sickle cell disease (SCD).
In June 2023, Jasper dosed the first patient in a Phase I clinical trial of briquilimab to treat LR-MDS.





