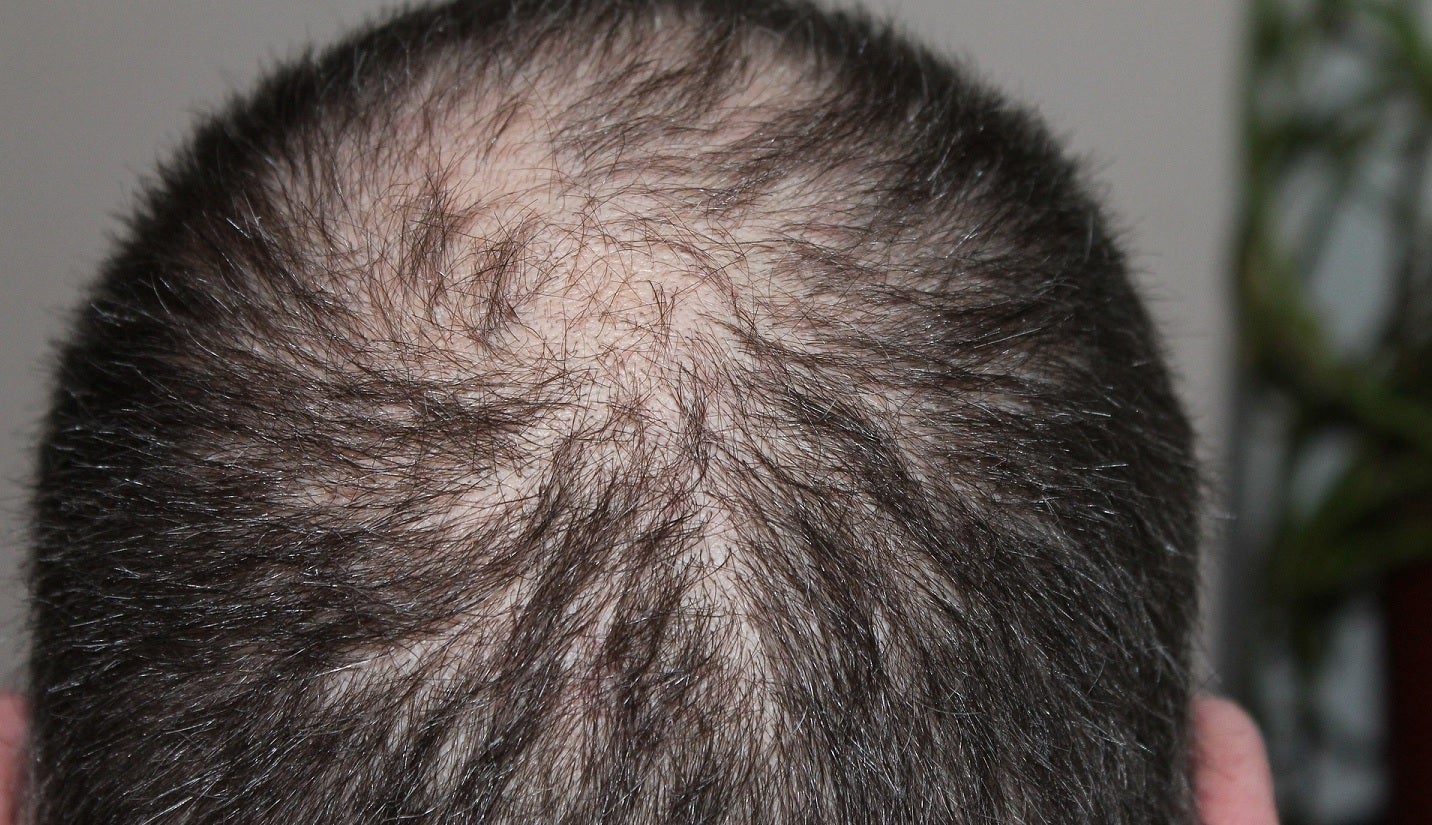
Kintor Pharmaceutical has reported positive top-line data from the US Phase I clinical trial of its proteolysis targeting chimera (PROTAC) compound, GT20029.
The placebo-controlled, parallel-group, randomised, double-blind, dose-escalation Phase I clinical trial assessed GT20029’s tolerability, pharmacokinetics, and safety after topical single ascending dose (SAD) administration in healthy participants and multiple ascending dose administration (MAD) in androgenetic alopecia (AGA) or acne subjects.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Findings from the trial demonstrated that GT20029 was well tolerated, safe, and had good pharmacokinetic characteristics in healthy and AGA or acne subjects.
It was also found to be well tolerated and safe in all cohorts at all dose levels.
Burning, dryness, itching and pain at the application site were some of the most common treatment-emergent adverse events observed in the MAD stage.
Kintor Pharmaceutical founder, chairman and CEO Dr Youzhi Tong said: “The positive US Phase I top-line results of GT20029 in 123 subjects has shown a similar result with that of Phase I trial in China with 92 subjects enrolled.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Both studies have demonstrated the good safety and tolerability in MAD of GT20029. Alopecia affects about 1.6 billion people and acne affects about 0.72 billion people worldwide, there are huge unmet clinical needs.
“We will accelerate the initiation of Phase II clinical trial of GT20029, and maintain our leading position in the development of topical PROTAC drug candidate globally.”
Last August, Kintor concluded subject enrolment in the double-blind, parallel-group, randomised, placebo-controlled Phase II clinical trial of KX-826 (pyrilutamide) to treat male androgenetic alopecia (AGA) patients in the US.





