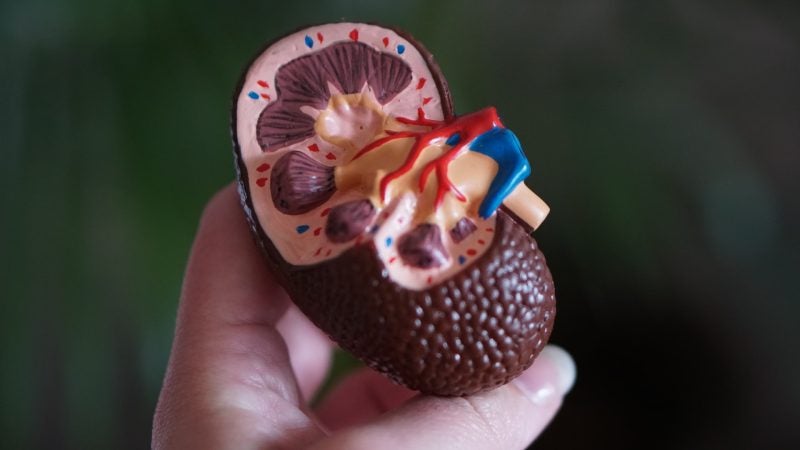
Kira Pharmaceuticals has received clearances in China and Australia to commence Phase II clinical trials of investigational bifunctional biologic, KP104, for IgA nephropathy (IgAN) and complement 3 glomerulopathy (C3G).
The Chinese National Medical Products Administration (NMPA) and the Australian Therapeutic Goods Administration (TGA) granted the approval.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The trials will assess the safety, efficacy, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of KP104 in IgAN and C3G patients in China and Australia.
Created to hinder the alternative and terminal complement pathways at the same time, KP104 has a dual-target mechanism of action.
Kira CEO Frederick Beddingfield said: “These clearances add to the multiple INDs Kira has secured this year for KP104 and mark our first in IgAN and C3G, serious immune-mediated conditions that cause kidney damage and often result in kidney failure.
“We believe that KP104’s ability to simultaneously and synergistically block two key complement targets makes it a unique therapeutic option with the potential to make a profound impact on the lives of patients living with these kidney diseases around the world.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to the findings from the Phase I SYNERGY-1 trial, KP104 showed clinical proof of mechanism.
Earlier this year, the US Food and Drug Administration (FDA) granted Orphan Drug Designation for the biologic to treat paroxysmal nocturnal haemoglobinuria (PNH).
At present, KP104 is being analysed in a Phase II study in PNH and the company has plans to analyse it for various haematology and nephrology indications.
An autoimmune ailment, IgAN damages the kidneys while C3G comprises dense deposit disease and C3 glomerulonephritis, which leads to glomeruli inflammation and damage in the kidney.



