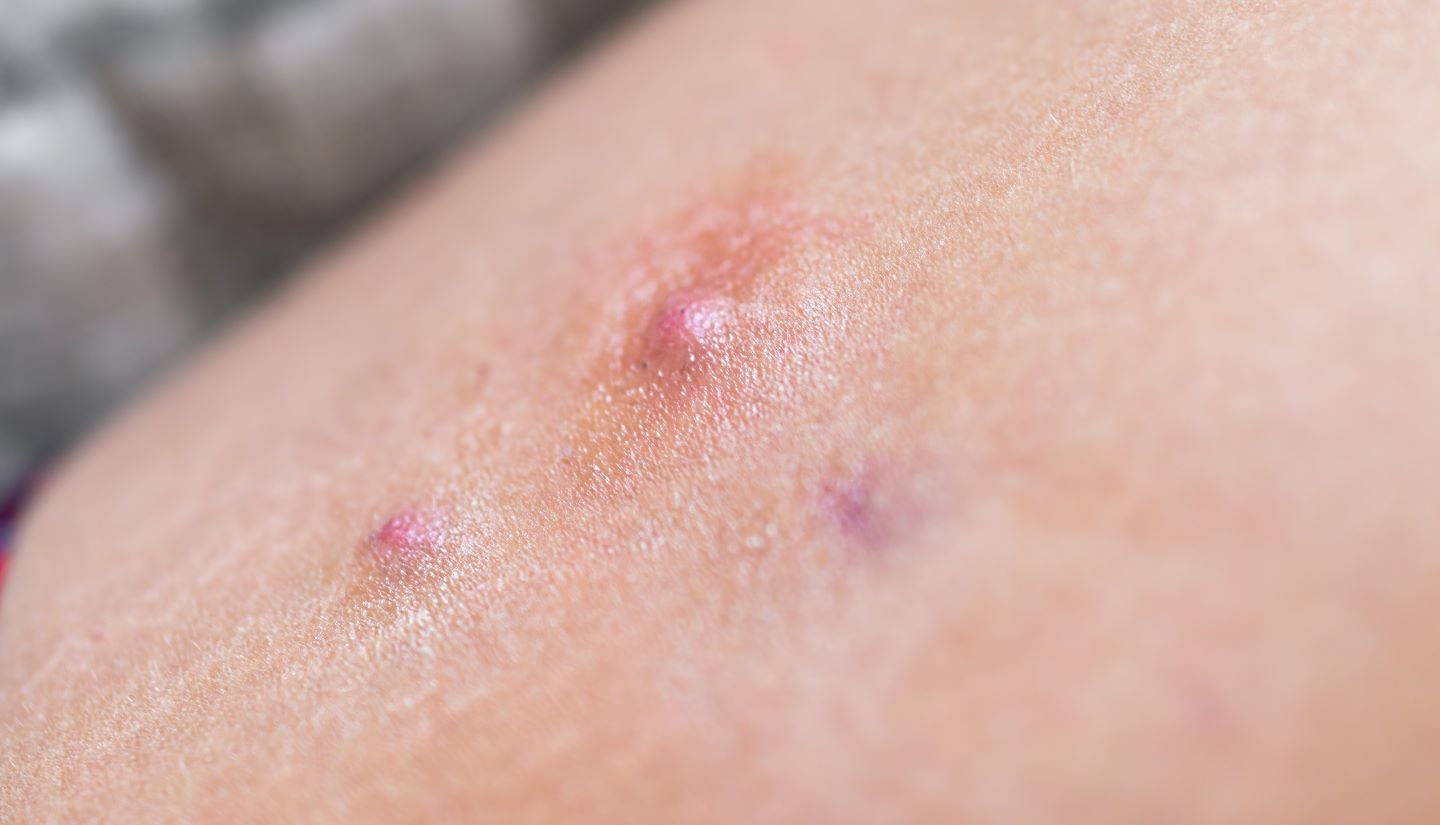
Kymera Therapeutics has announced the dosing of the first subject in the Phase II clinical trial of KT-474 (SAR444656) to treat hidradenitis suppurativa (HS), a chronic inflammatory skin ailment.
The randomised trial will assess the safety, efficacy, pharmacokinetics and biological effects of KT-474 versus placebo in adults with moderate to severe HS.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Under a partnership deal with Sanofi, Kymera received $40m in milestone payment following the initiation of subject dosing in the HS trial.
Currently, Sanofi is carrying out the Phase II study in HS and has also commenced a second randomised Phase II trial in atopic dermatitis (AD) patients.
The dosing of the first subject in the AD trial is anticipated later this quarter.
Subsequent to the dosing in this AD trial, Kymera will also receive an additional milestone payment from Sanofi.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataKymera Therapeutics founder, president and CEO Nello Mainolfi said: “The initiation of dosing in the first Phase II trial of KT-474 in HS is an important step in the development of this molecule and a significant achievement for Kymera in demonstrating the potential of protein degradation to transform the treatment of complex, inflammatory diseases with small molecules.
“Based on the encouraging KT-474 Phase I results, we believe that this molecule has the potential to offer HS patients a well-tolerated and effective oral drug.”
An oral degrader of interleukin 1 receptor-associated kinase 4 (IRAK4), KT-474 is being developed to treat various complex inflammatory ailments.



