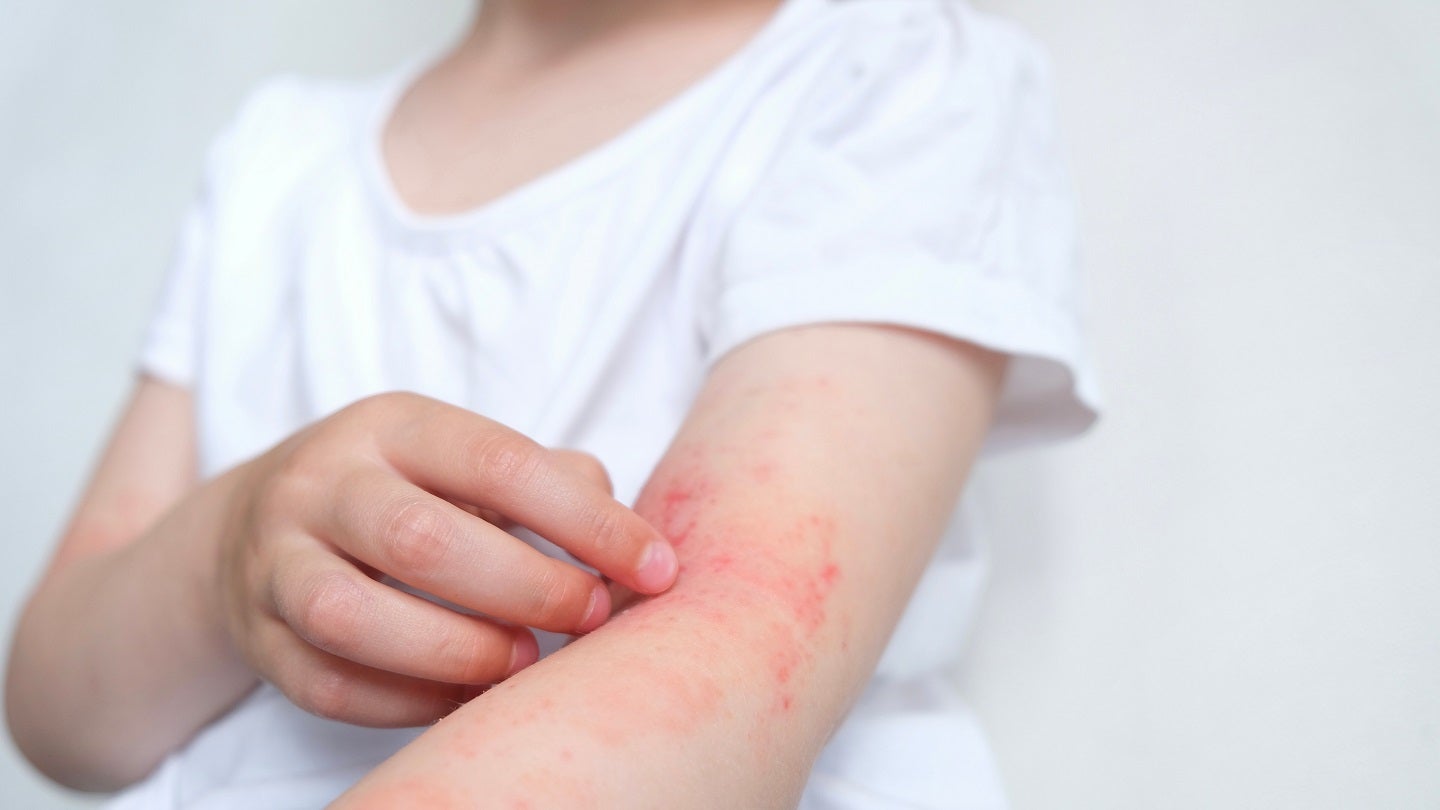
Kymera Therapeutics has reported positive results from a Phase I clinical trial of its lead programme, KT-474 (SAR444656), for the treatment of hidradenitis suppurativa (HS) and atopic dermatitis (AD).
KT-474 is a degrader of IRAK4, a protein that activates toll-like receptors (TLRs) and IL-1 receptors (IL-1Rs) causing inflammation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It blocks TLR/IL-1R-mediated inflammation more effectively compared to monoclonal antibodies targeting single cytokines.
KT-474 also abolishes both the kinase and scaffolding functions of IRAK4 and is said to be superior to IRAK4 kinase inhibitors.
In a Phase I trial, KT-474 demonstrated robust IRAK4 degradation in skin and blood lesions and showed systemic anti-inflammatory effect in HS and AD patients.
KT-474 was also found to be well-tolerated and showed similar safety, pharmacokinetics and pharmacodynamics properties as showed by healthy volunteers.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataA highly competitive profile with substantial responses in majority of patients with moderate-to-severe HS and AD was also observed in the study.
Kymera Therapeutics chief medical officer Jared Gollob said: “KT-474 exhibited clinical activity in HS and AD patients that we believe is superior to IRAK4 small molecule kinase inhibitors and appears competitive with standard of care biologics and other agents in development in both HS and AD.
“These results validate IRAK4 degradation as a potential best-in-class mechanism in TLR/IL-1R-driven inflammatory diseases that has the potential to improve the lives of patients with conditions like HS and AD and potentially other indications.
“We look forward to sharing additional updates as our partner Sanofi initiates Phase II clinical trials of KT-474 in HS and AD, with the first study in HS planned for initiation in 2023.”





