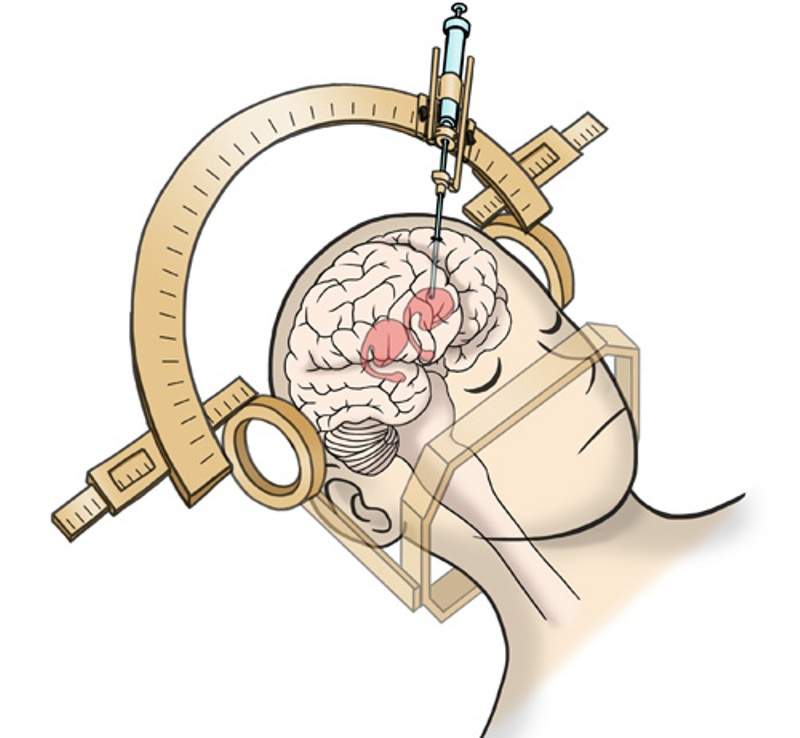
Kyoto University’s Centre for iPS Cell Research and Application (CiRA) in Japan is to start two clinical trials that will evaluate human induced pluripotent stem cells (iPS) to treat Parkinson’s disease.
One of the Kyoto trials will evaluate the safety and efficacy of iPSC-derived dopaminergic progenitors to treat Parkinson’s.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This Phase I/II trial aims to investigate the safety and efficacy of transplanting human iPS cell-derived dopaminergic progenitors into the putamen of Parkinson’s disease patients.
The other trial will evaluate the safety and efficacy of tacrolimus in the iPSC-based therapy to treat Parkinson’s disease is a Phase III study.
It intends to examine the safety and efficacy of tacrolimus for Parkinson’s disease patients who were transplanted with human iPS cell-derived dopaminergic progenitors into their putamen.
Scheduled to begin tomorrow, both trials are expected to transplant dopaminergic progenitors generated from iPS cells. The aim is to restore normal production of the neurotransmitter dopamine, which the absence of in the brain leads to Parkinson’s disease.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLack of dopamine primarily affects the patients’ motor system, leading to walking difficulties and unintentional trembling. As the disease advances, it can cause dementia.
CiRA professor Jun Takahashi was quoted by Reuters as saying: “This will be the world’s first clinical trial using iPS cells on Parkinson’s disease.”
In a statement cited by Reuters CiRA head Yamanaka said: “We intend to carry on conducting our research carefully, yet expeditiously, in coordination with Kyoto University Hospital, so that new treatment using iPS cells will be brought to patients as soon as possible.”
Shinya Yamanaka won the Nobel Prize for medicine in 2012 along with a British scientist John Gurdon for the discovery that adult cells can be altered to become embryo-like types.





