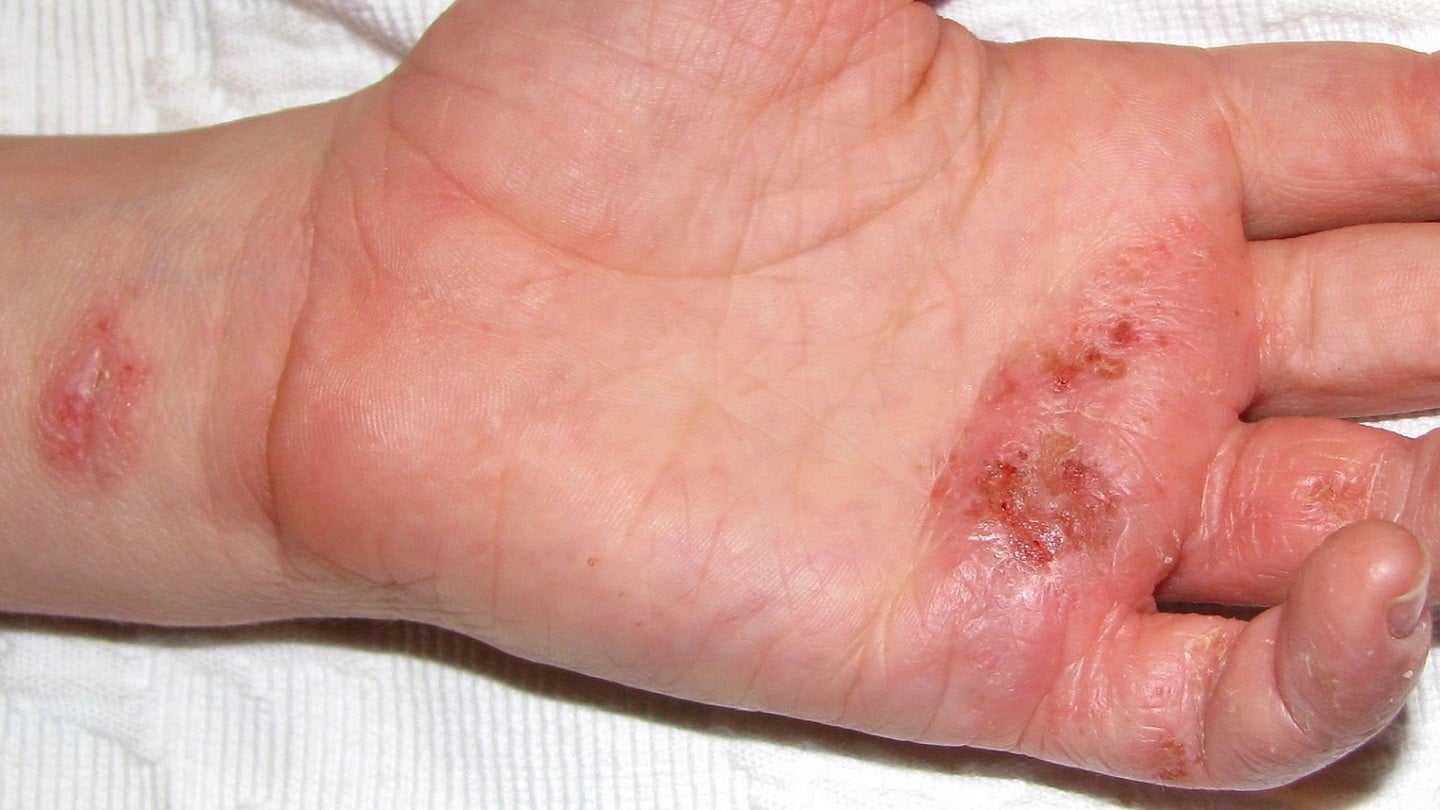
LEO Pharma has reported positive data from the DELTA 2 trial of delgocitinib cream in moderate to severe chronic hand eczema (CHE).
The second of two pivotal Phase III clinical trials, the DELTA 2 trial has been designed for evaluating the efficacy of twice-daily delgocitinib cream against cream vehicle for a 16-week treatment period in adult patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Findings showed that the trial met its primary and important secondary endpoints, which confirmed the positive results of the DELTA 1 trial.
Patients treated with delgocitinib cream showed statistically significant improvement in CHE after 16 weeks. It was also found to be well-tolerated.
The cream also provided efficacious treatment and relief in early CHE symptoms.
Further analyses will be conducted for determining the complete potential of the delgocitinib cream in moderate to severe CHE adult patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLEO Pharma Global Research & Development executive vice-president Jörg Möller said: “It is incredibly exciting to see the level of consistency that our DELTA 2 results show in line with the positive DELTA 1 results announced late last year.
“CHE is a condition that we know can have a hugely negative impact on patient quality of life, physical functioning, and ability to work.
“These results bring us one step closer towards establishing delgocitinib as a best-in-class innovative topical treatment for patients affected by this hard-to-treat disease.”
The company stated that the participants who have completed the 16-week treatment in the DELTA 1 or DELTA 2 trials are offered to roll-over to the DELTA 3 extension trial for assessing the cream’s long-term effects.
It intends to later submit the detailed data from the DELTA 2 trial for scientific presentation and publication.





