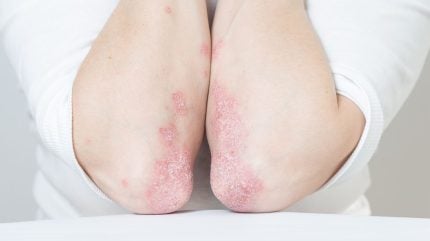
LEO Pharma has announced positive data from the Phase III clinical trial of Enstilar (LEO 90100) in Chinese adult patients with stable plaque psoriasis.
An aerosol spray foam, enstilar combines 50mcg/g calcipotriol monohydrate and 0.5mg/g betamethasone dipropionate.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is an advanced formulation of the existing treatment, Daivobet ointment.
The multicentre, randomised, investigator-blind, active-controlled, parallel-group trial compared the safety and efficacy of the once-a-day application of Enstilar with Daivobet ointment over four weeks.
A total of 604 patients across 39 sites in China participated in the study.
The primary goal of the trial was to assess the efficacy of Enstilar versus Daivobet on stable plaque psoriasis severity and extent.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAssessment of treatment safety was the trial’s secondary objective while exploratory objectives looked at the impact on health-related quality of life.
The trial concluded recruitment four months ahead of schedule, and the topline results have shown that Enstilar outperformed the ointment in achieving the primary objective.
Furthermore, Enstilar demonstrated superiority in both primary and secondary endpoints, indicating enhanced efficacy in reducing the severity and extent of stable plaque psoriasis.
Both Daivobet ointment and Enstilar, which are products of LEO Pharma, were found to be well tolerated in the trial.
Furthermore, the safety profiles of both treatments were in line with previous trials without any new safety concerns identified.
Based on the latest data, LEO Pharma is preparing to submit Enstilar for securing approval from the Chinese authorities.
LEO Pharma global product strategy and international operations executive vice-president Becki Morison said: “The results of this trial are very encouraging for LEO Pharma’s presence in China. Not only do we see superiority in the results, but we also see that both portfolio products were efficacious and well-tolerated.
“This is a key market for LEO Pharma – with dermatology growing rapidly in the country and our revenue steadily increasing through a strong performance from our team in China. This trial is a good example of how LEO Pharma is leveraging our deep dermatology knowledge to expand both our innovative and core portfolios in China.”
Last October, the company reported positive data from the Phase III DELTA 3 trial of delgocitinib cream to treat adult patients with moderate to severe chronic hand eczema.





