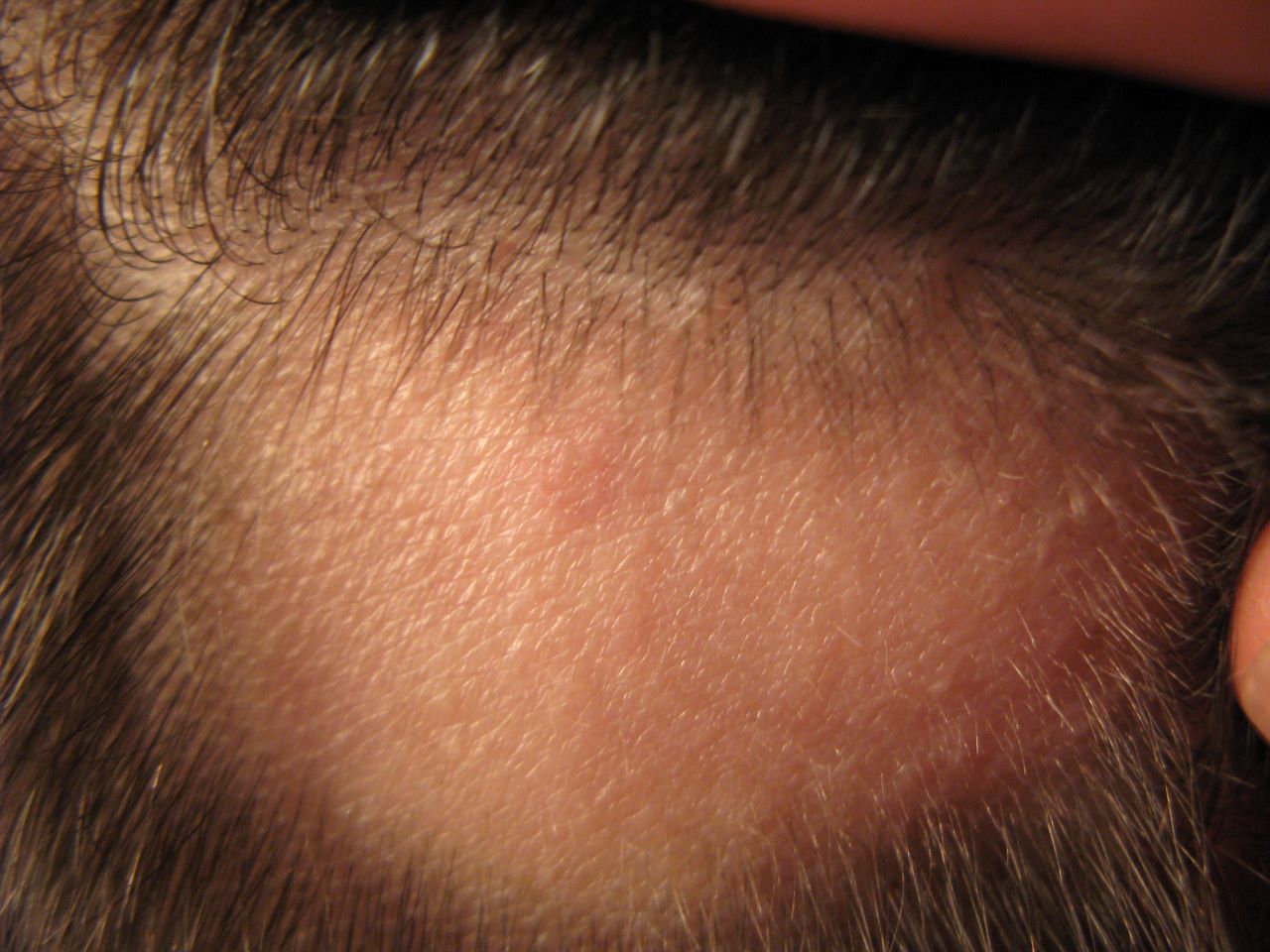
Eli Lilly and Company and Incyte have reported positive top-line results from Phase III BRAVE-AA2 study of baricitinib, which demonstrated hair regrowth in adults with severe alopecia areata (AA).
Discovered by Incyte and licenced to Lilly, baricitinib is a once-daily, oral JAK inhibitor. It is sold as OLUMIANT in the US and over 70 countries for treating adults with moderate to severe rheumatoid arthritis (RA).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
An autoimmune disease, AA causes patchy hair loss on the scalp, face and other areas of the body.
The multi-centre, randomised, double-blind, placebo-controlled study analysed the efficacy and safety of a once-daily 2mg or 4mg dose of baricitinib in 546 adults with scalp hair loss of 50% or more.
The subjects also had a recent severe AA episode which lasted at least six months and not more than eight years.
A diverse population of patients from Argentina, Australia, Brazil, China, Israel, Japan, South Korea, Taiwan and the US were part of the study.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAt week 36, data showed that both doses of baricitinib demonstrated statistically significant improvement in scalp hair regrowth versus placebo, thereby meeting the primary efficacy endpoint.
Furthermore, baricitinib’s safety outcomes in the trial were in line with the already established safety profile in RA and atopic dermatitis patients.
No deaths, major adverse cardiovascular events (MACE) or venous thromboembolic events (VTEs) were reported in the study.
Eli Lilly immunology development vice-president Lotus Mallbris said: “For patients who suffer from alopecia areata, it is not a cosmetic condition, it is a devastating autoimmune disease that can have significant psychological effects. They lose much more than just hair.
“We are looking forward to sharing the totality of data from the overall clinical development programme for baricitinib as a potential first-in-disease treatment for alopecia areata.”
Last December, the National Institute of Allergy and Infectious Diseases (NIAID) in the US announced results from a Phase III study which show how baricitinib plus remdesivir reduced recovery time for people hospitalised with Covid-19.





