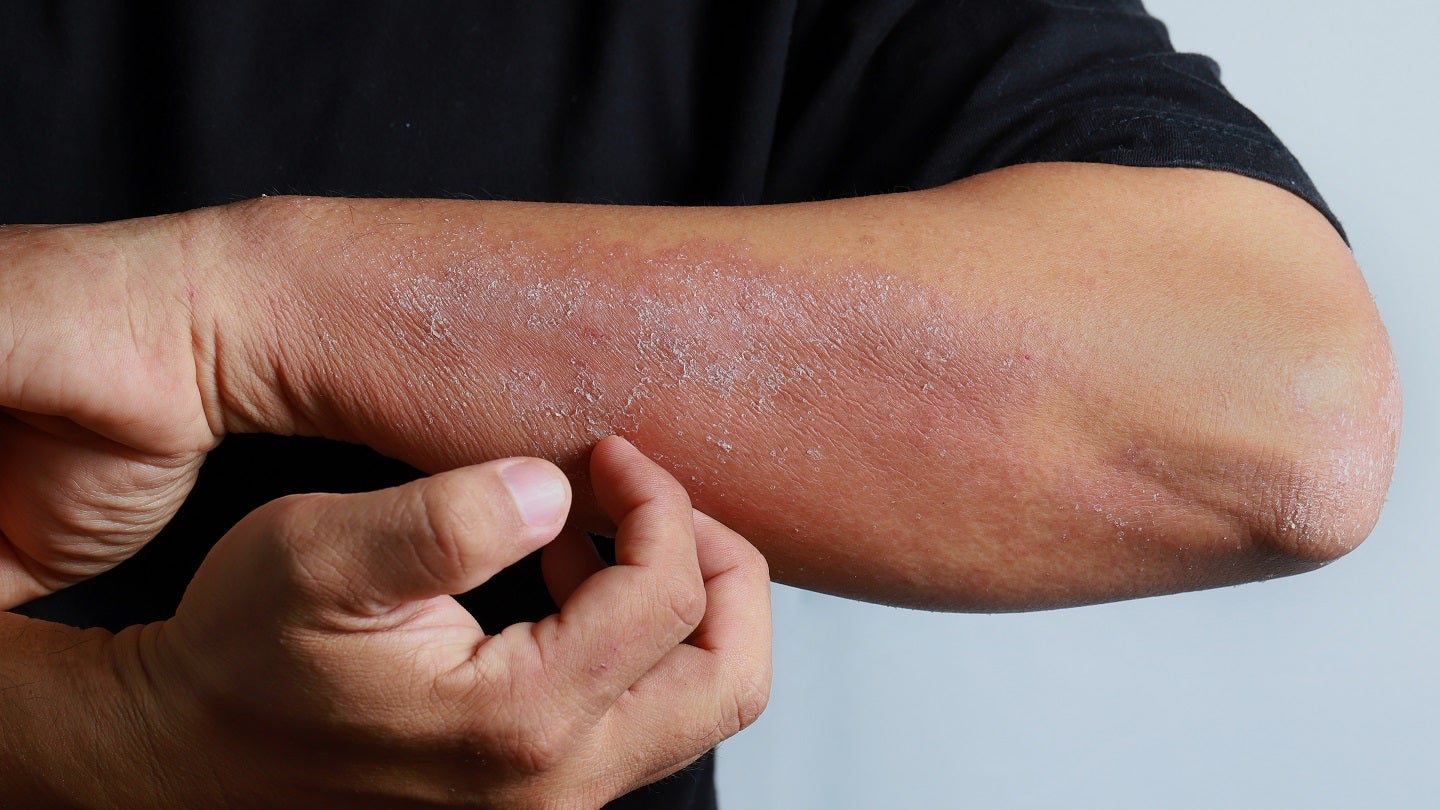
Lynk Pharmaceuticals has reported positive topline data from a Phase II clinical trial of LNK01001 to treat atopic dermatitis (AD).
The multicentre, double-blind, randomised, placebo-controlled study was led by Peking University People’s Hospital Dermatology department director professor Jianzhong Zhang.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It enrolled a total of 150 adult patients aged 18 to 75 years, with moderate-to-severe AD, who previously received systemic treatment or did not respond well to topical treatment for AD.
They were randomised into a 1:1:1 ratio to receive a high-dose or low-dose of LNK01001 or a placebo.
Percentage change from baseline in the eczema area and severity index (EASI) score, determined at week 12, is the trial’s primary efficacy endpoint.
In preliminary studies, the data showed a significant difference in the percentage change from baseline in EASI score in patients who completed 12 weeks of treatment, compared to the placebo.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIn addition, the proportion of responders achieving Investigator’s Global Assessment response and EASI-75 were higher in both treatment groups, in comparison to the placebo group.
A rapid onset of improvement in pruritus was observed in both groups, whereas the placebo group showed improvement 24 hours after dosing.
In terms of safety, overall tolerability was good in both groups, with comparable rates of CTC grade 2+ treatment-emergent adverse events and serious adverse events.
No severe infections, malignancies, venous thromboembolism, or major adverse cardiovascular events were observed.
Lynk Pharmaceuticals chief development officer Dr Henry Wu said: “With social development and lifestyle changes, the number of atopic dermatitis patients is increasing, with a global population of 390 million.
“We are pleased to see that LNK01001 has shown positive results in the treatment of atopic dermatitis.
“We are preparing to submit an application for the end of Phase II (EOP2)/Pre-Phase III meeting to advance the clinical development of this compound and strive to benefit more patients as soon as possible.”





