MediciNova has reported positive results from the SPRINT-MS Phase llb trial that examined the safety, tolerability of MN-166 (ibudilast) for the treatment of progressive multiple sclerosis (progressive MS).
The trial enrolled 255 patients with primary progressive or secondary progressive multiple sclerosis (PPMS and SPMS) at 28 clinical sites across the US.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The PPMS patients were either untreated with long-term disease-modifying therapy (DMT) or being treated with either glatiramer acetate (GA) or interferon beta (IFNβ-1a or IFNβ-1b).
As part of the trial, the patients were randomly distributed in 1:1 ratio to administer a twice-daily oral dose of MN-166 (ibudilast) up to 100mg/day or inactive control (placebo).
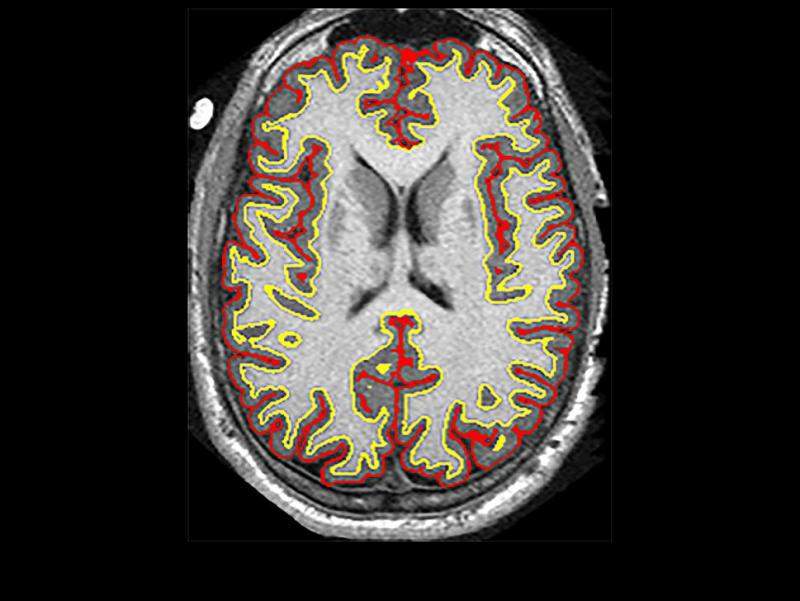
The SPRINT-MS’ primary objectives were to analyse the activity of ibudilast (MN-166) against placebo at 96 weeks as measured by quantitative magnetic resonance imaging (MRI) analysis for whole brain atrophy using brain parenchymal fraction (BPF).
The trial also examined the safety and tolerability of ibudilast (MN-166) in comparison with placebo in the enrolled subjects.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial’s additional measures comprise disability, imaging analyses of brain and retinal tissue integrity, as well as cortical atrophy, cognitive impairment, quality-of-life and neuropathic pain.
Its exploratory objectives were pharmacokinetic and biomarker analyses.
Results from the trial showed that ibudilast was better than placebo in reducing brain shrinkage.
They also showed that the most common side effects of ibudilast were gastrointestinal, including nausea and diarrhea, in addition to headaches and depression.
SPRINT-MS trial principal investigator Robert Fox said: “These findings are significant for patients with progressive MS.
“Our hope is that the benefit of ibudilast in slowing brain shrinkage will also translate to decreased progression of associated physical disabilities in a future Phase lll trial.”
The SPRINT-MS trial received a portion of its funding from the US National Institutes of Health (NIH) and support from NeuroNEXT, Cleveland Clinic and others.





