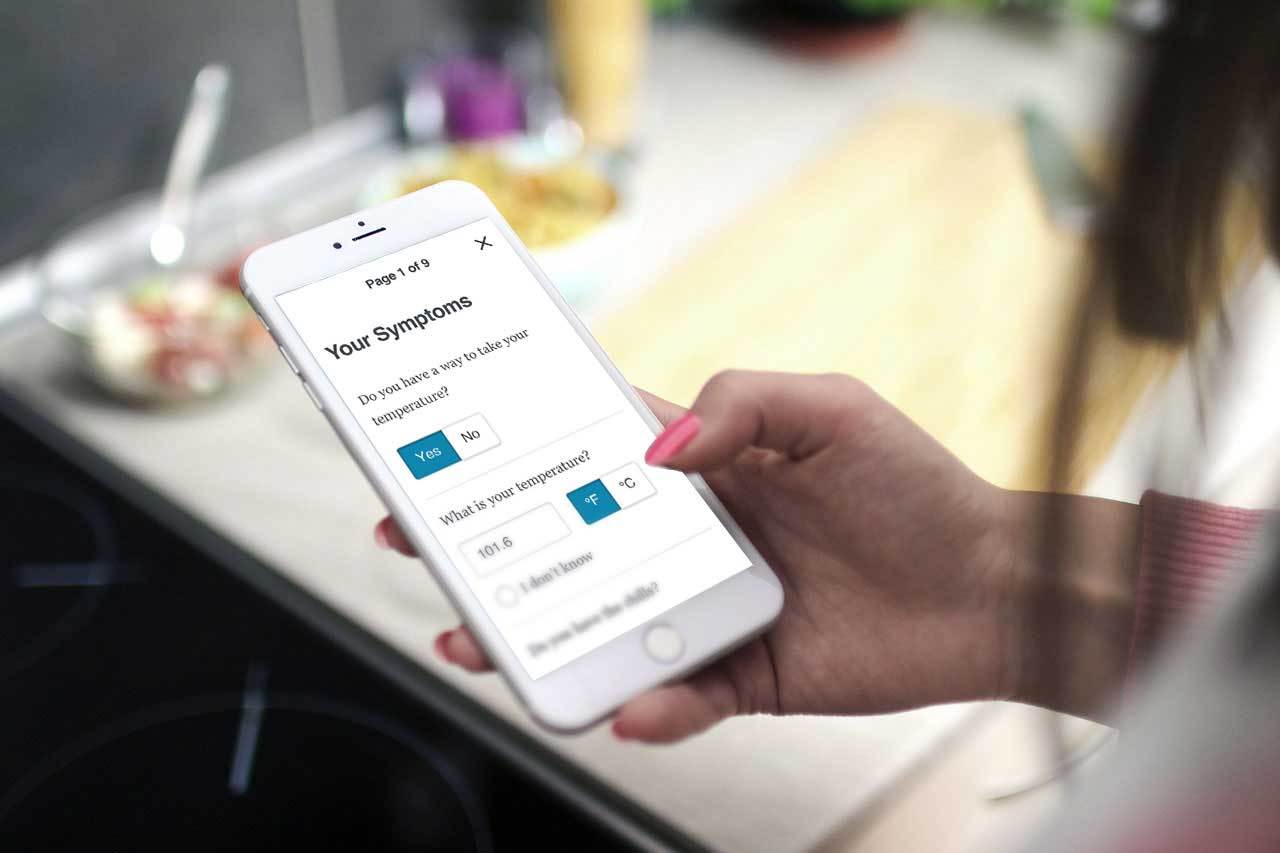
American technology firm Medidata, a subsidiary of Dassault Systèmes, has launched an advanced, intuitive platform called myMedidata for patients.
The myMedidata platform will enable flexible clinical trials participation for new vaccines.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is designed to support research studies and speed up scientific understanding of the virus. Its first release includes a research-based Covid-19 symptom tracker.
The technology firm noted that the app will provide sponsors the ability to collect symptoms directly from research participants who may otherwise not be able to continue with traditional site visits.
This app also allows researchers to enrol huge registries of individuals to monitor their health status with respect to coronavirus.
Medidata co-founder and co-CEO Glen de Vries said: “It is critical for the life sciences industry to understand the effects of SARS-CoV-2 on patients, and to find ways to forge ahead with research across all therapeutic areas, from Covid-19 to cancer.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The early launch of myMedidata allows sponsors to gather patient-reported Covid-19 symptoms in ongoing and new research programmes.”
The intuitive platform provides a comprehensive, integrated tool set for all aspects of patient-centric research.
The tool set includes eConsent, an electronic patient consent system for clinical trial participation, an electronic clinical outcomes assessment (eCOA), and an electronic patient-reported outcomes (ePRO).
Also included are wearable sensors that collect data from biosensors and wearable technology, as well as virtual trials.
With the help of myMedidata platform, patients will be able to view their own clinical data, both current and historical. It also helps increase their engagement with the study team.
The platform was built using insights generated by Medidata’s ‘Patient Centricity by Design’ framework.
Medidata mHealth senior vice-president Anthony Costello said: “Therapeutics and vaccines cannot be tested without the most critical components in clinical research, the patients, and their ability to participate.
“Making research more patient-centric and giving patients the ability to virtually access and actively engage in their own health care needs to be the new normal.”
In February, Medidata and Project ALS entered a research partnership in order to develop new therapeutic strategies for the treatment of Amyotrophic lateral sclerosis (ALS).





