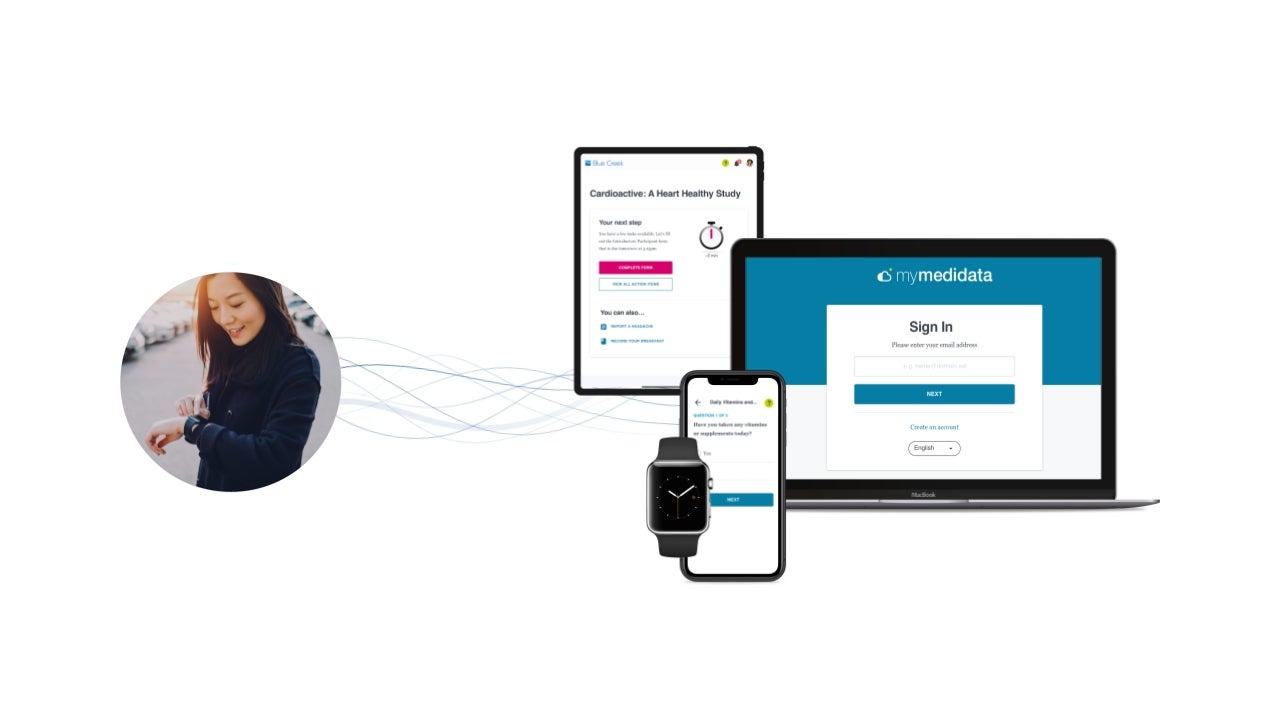
Medidata, a Dassault Systèmes subsidiary, has launched its Decentralized Clinical Trials (DCT) Program, which is a suite of technologies to completely decentralise the clinical trial continuum.
The platform can be used by trial sponsors and contract research organisations (CROs).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It integrates technology and workflows to virtualise the participation of patients. The platform also features tools that allow sponsors to manage patient safety and data quality.
In addition, the new programme facilitates direct-to-patient services, such as the delivery of study drugs to patients’ homes.
The Medidata DCT Program is designed to remotely capture participant data in any place at any time. Subsequently, the platform combines and transforms this data.
It tracks the data to detect quality issues to lower risk and aid patient safety. Furthermore, analytics are applied for a better understanding and improved outcomes for patients, researchers, sites, sponsors, and CROs.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe company designed the platform to facilitate ‘on’ and ‘off’ of its capabilities in different combinations. This can be achieved using the Trial Dial concept to customise decentralising solutions depending on the study protocol.
Study sponsors can use this feature to select standard onsite trials, fully decentralised models or any hybrid trial design among others.
Medidata Patient Cloud president Anthony Costello said: “We are very proud to say that, as a trusted partner to the life science sector for more than 20 years, Medidata is now the only company providing a full suite of virtual capabilities to enable complete trial decentralisation, encompassing both patient and site interactions.
“The DCT Program marks an important evolution in Medidata’s vision for how we can better serve patients and customers, by accelerating research and bringing novel therapies to market in record time.”
The company has adopted its decentralising technologies at more than 44,000 clinical sites in several languages, including more than 600,000 patients with various diseases globally, so far.
Medidata added that the new single platform lowers the risk for data inconsistencies and transfer delays, which may cause security concerns and trial interruption.
Last month, the company introduced a clinical endpoint adjudication system integrated with its Medidata Clinical Cloud.





