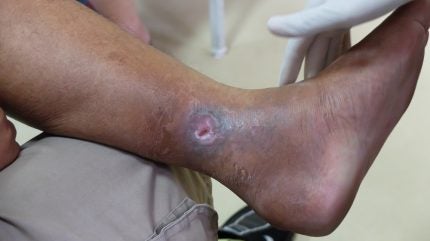
Israel-based MediWound has announced positive results from a head-to-head comparison of its wound treatment EscharEx versus standard of care (SoC) Santyl – a collagenase ointment – in venous leg ulcers (VLUs).
MediWound’s lead asset EscharEx is a bioactive, multimodal debridement therapy that can be applied topically and is currently in the advanced stages of clinical development. The candidate acts by removing eschar [dry dead tissue] from the wounds. The mechanism of action is mediated by proteolytic enzymes that cleave to and remove necrotic tissue to prepare the wound bed for healing.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A secondary analysis from the Phase II ChronEx study showed superiority in incidence and time to complete debridement, complete granulation, and wound closure in patients treated with EscharEx compared to a sub-group of patients who were treated with market leader Santyl (collagenase clostridium histolyticum).
The analysis further supports MediWound’s upcoming Phase III trial, which is set to begin in the second half of 2024.
“Given the exceptionally strong data from our Phase II study, and the fact that we already have received approval for another drug – NexoBrid – with the same API and a similar indication, we believe EscharEx has an excellent chance at receiving FDA approval,” Mediwound CEO Ofer Gonen told Clinical Trials Arena.
The Phase III study will take place in three stages, an initial debridement phase of two weeks and a wound healing phase of ten weeks, then a three-month follow-up to assess wound recurrence.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThere will be 216 patients randomised on a 1:1 ratio of EscharEx versus placebo. Primary endpoints are complete wound debridement and complete wound closure.
EscharEx superior at Phase II
In the direct comparison, the incidence of complete debridement over two weeks of treatment was 63% for EscharEx and 0% for Santyl. The average time to complete debridement during the study was nine days for EscharEx while this endpoint was not achieved with Santyl.
Complete debridement and complete cover of the wound bed preparation (WBP) with granulation tissue during the daily treatment period was 50% for EscharEx compared to 0% for Santyl. The average time to WBP was 11 days with EscharEx while this was not achieved with Santyl.
Of the 46 patients treated with EscharEx, 15 completely closed their wounds during the study, compared to two out of eight patients treated with Santyl.
In the ChronEx study, patients with chronic VLU were randomised to daily treatment with EscharEx placebo, or non-surgical SoC for up to 2 weeks or until reaching complete debridement and then treated with non-surgical SoC for 12 weeks. The non-surgical SOC arm included Santyl, hydrogels, medical-grade honey, and non-active dressings.
GlobalData’s Pharmaceutical Intelligence Centre reports that EscharEx is also being investigated in basal cell carcinoma, wounds and diabetic foot ulcers. The investigational candidate has a predicted global sales forecast of £92m in 2029.
GlobalData is the parent company of the Clinical Trials Arena.





