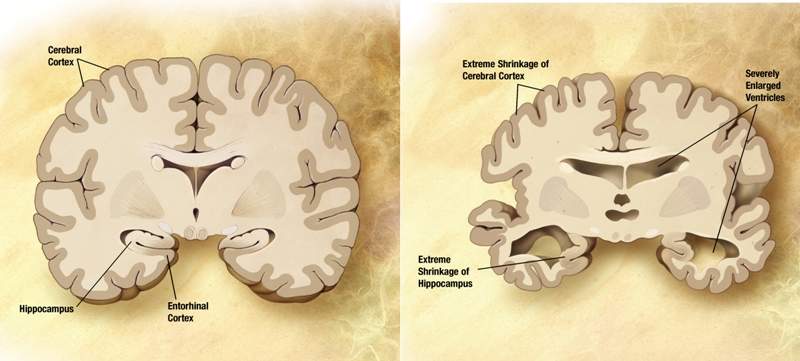
MGC Pharmaceuticals (MXC) has received approval from the University of Notre Dame in Western Australia’s (UNDA) Human Research Ethics Committee (HREC) to carry out a Phase II clinical trial to examine the effects of CogniCann for the treatment of patients with mild dementia and Alzheimer’s disease.
The approval was granted following the completion of an ethical review by HREC, in line with Australia’s National Statement on Ethical Conduct in Human Research rules.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Subject to Australia’s Therapeutic Goods Administration (TGA) approval, the 16-week, randomised, double-blind, crossover, placebo-control trial is expected to start early next year.
UNDA’s Institute for Health Research will conduct the trial, which is designed to investigate the behavioural changes, quality of life, and level of discomfort and pain in dementia patients staying at residential aged care facilities.
The trial aims to enrol a total of 50 subjects aged 65 and older.
In addition, MGC Pharmaceuticals plans to carry out a number of pre and posttreatment surveys and focus groups to evaluate the patients and their family member’s knowledge and perceptions towards the use of CogniCann.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataUniversity of Notre Dame Australia Institute for Health Research director Jim Codde said: “Health research, especially into issues affecting those most in need within our community, is of the highest priority to Notre Dame.
“Research initiatives into dementia is also a national priority, so we are very excited to work with MXC and the aged care sector to trial this novel approach to improve the quality of life for the almost 350,000 Australians suffering this disease that currently has no cure.”
CogniCann is part of MGC Pharmaceuticals’ medical cannabis pharmaceutical products featuring a THC:CBD ratio specifically developed for the treatment of major dementia symptoms and improve certain cognitive functions.





