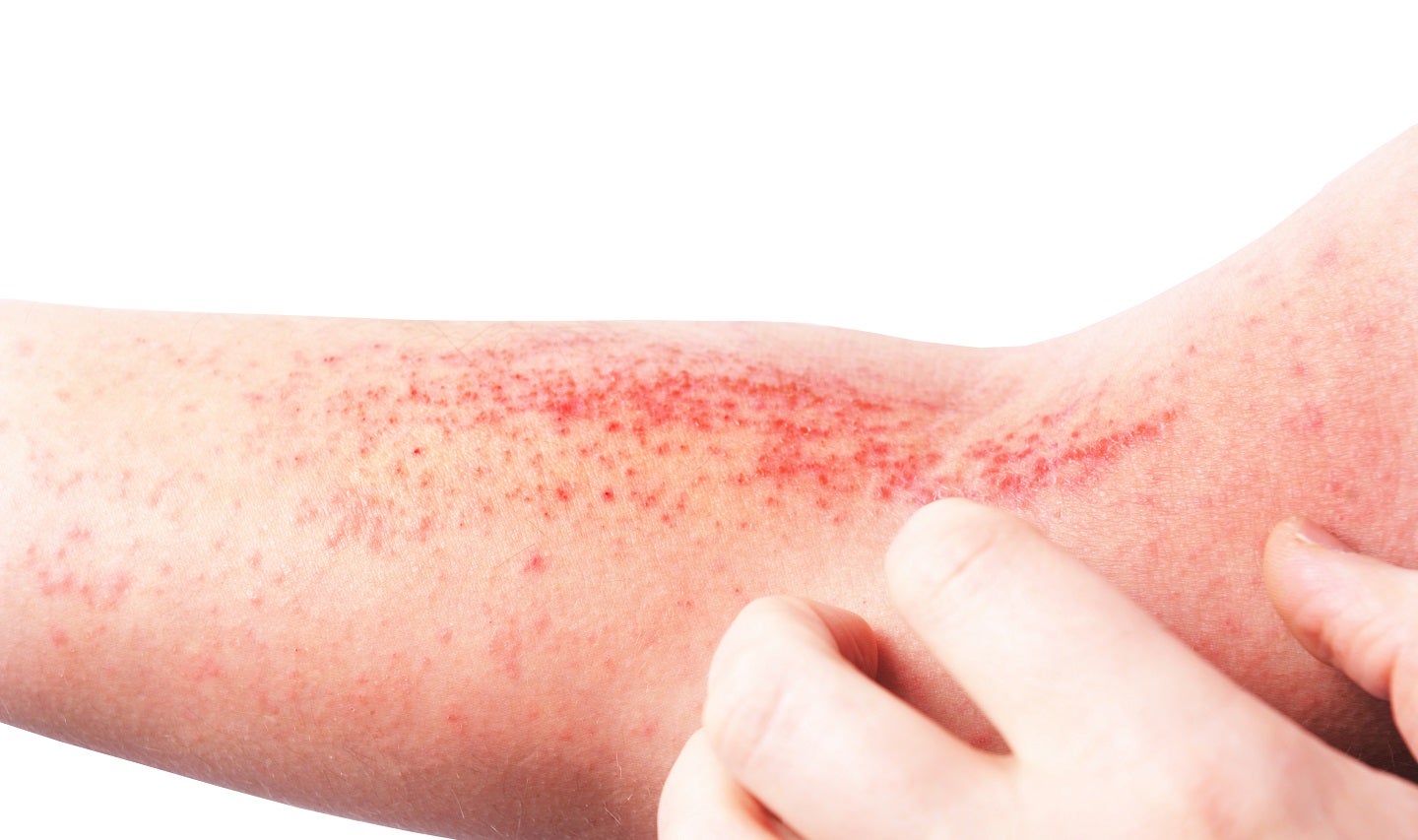
Minghui Pharmaceutical has announced top line positive data from its Phase II trial of MH004 Cream for the treatment of adolescents and adults with mild-to-moderate atopic dermatitis (AD).
Designed to assess the safety, tolerability, pharmacokinetics and efficacy of MH004, the multi-centre, double blind, vehicle-controlled proof-of-concept study evaluated two strengths of MH004 Cream including 0.3% or 1.0% for four weeks.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
For this trial, 150 adolescents aged 12 years and older and adults with mild-to-moderate AD involving 3% ~ 20% body surface area (BSA) at baseline were enrolled.
The trial met its primary endpoint of mean percentage changes from baseline in EASI score. It also met complete secondary endpoints including IGA-TS and Eczema Area and Severity Index (EASI)-75 response.
EASI-75 was achieved in 79.6% subjects treated with 1.0% MH004 cream versus 42.0% of individuals treated with vehicle.
Mean percentage change from baseline in EASI score was 78.7% in 1.0% MH004 treatment group compared to 46.7% in vehicle group.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMinghui Pharmaceutical CEO Dr Guoqing Cao said: “MH004 Cream is a topical cream containing a JAK inhibitor that was designed with Minghui’s proprietary technology and formulation.
“These unique designs significantly improved the penetration of low protein binding molecules into multiple skin layers, resulting in a much higher free drug concentration in local skin tissues and much reduced systemic exposure.
“We observed excellent clinical efficacy with reduced side effects and hence the best-in-class potential. Based on the current Phase II results, we plan to further pursue the development of MH004 Cream in atopic dermatitis as well as other dermatological diseases.”





