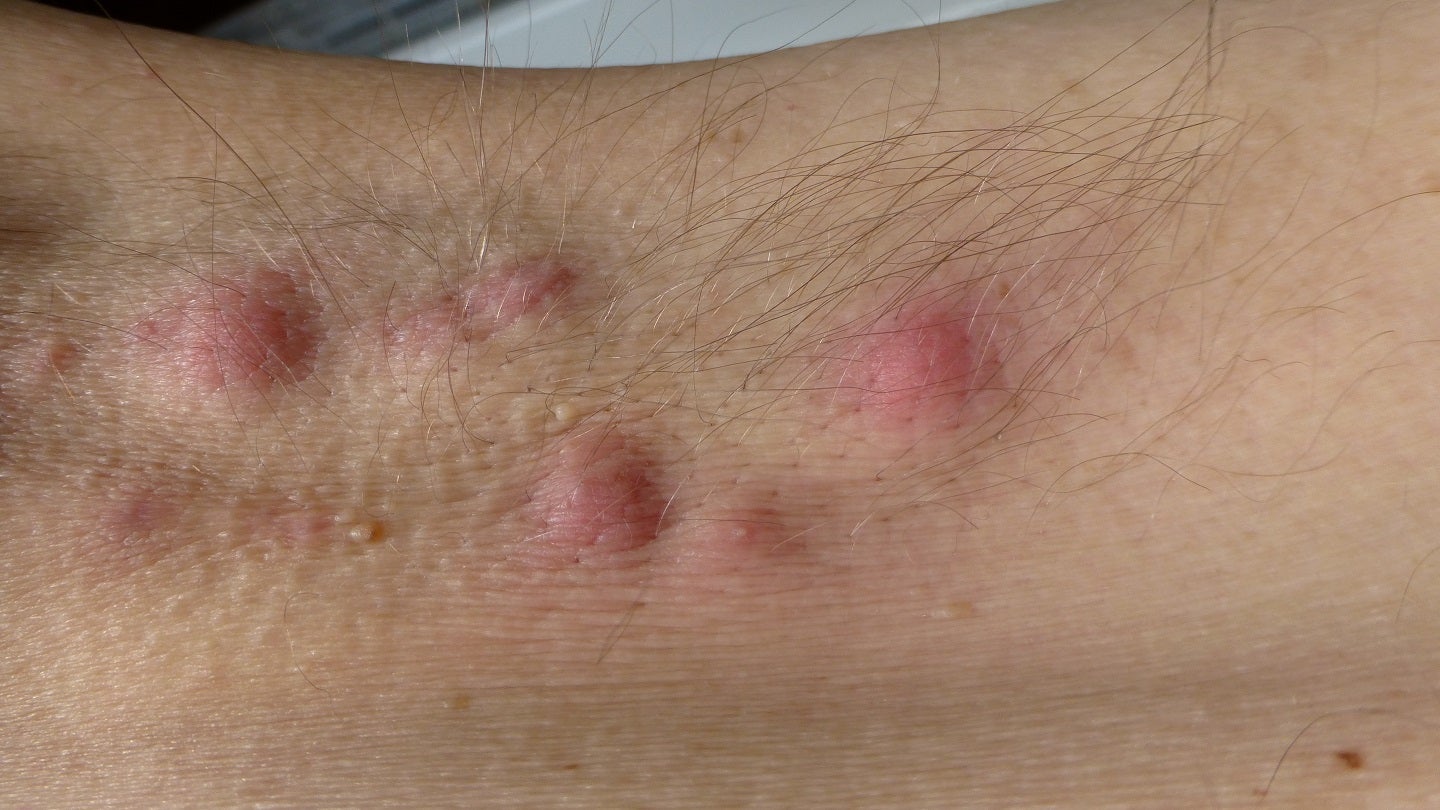
MoonLake Immunotherapeutics has reported positive top-line results from the Phase II MIRA trial of Nanobody sonelokimab for the treatment of hidradenitis suppurativa (HS), a chronic, inflammatory, skin disease.
The placebo-controlled, global, double-blind, randomised trial is designed to assess the safety and efficacy of sonelokimab in adult patients with active moderate-to-severe HS.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It enrolled 234 patients who were administered subcutaneously with sonelokimab 120mg and 240mg against placebo. Adalimumab was used as an active reference arm.
The trial met its primary endpoint achieving Hidradenitis Suppurativa Clinical Response (HiSCR) 75 for both the dosages against placebo at week 12.
Secondary endpoints were also met at week 12, including improvements in International Hidradenitis Suppurativa Severity Score System (IHS) 4, and HiSCR90.
Improvements in quality of life outcomes and abscess/nodule and draining tunnel counts were also observed.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe 120mg dose provided the highest delta of 29ppt and 38ppt on HiSCR75 for HiSCR50, respectively.
A favourable safety profile of sonelokimab was observed and no new safety signals were reported.
MoonLake founder and chief scientific officer Kristian Reich said: “Importantly, the results confirm the advantage of the Nanobody’s smaller size versus traditional antibodies in the treatment of diseases in which high-level improvements depend on optimal tissue penetration such as hidradenitis suppurativa and likely psoriatic arthritis.”
Sonelokimab also showed durable skin clearance in patients with moderate-to-severe plaque-type psoriasis in a Phase IIb trial.
MoonLake is also planning to conduct a four-week safety follow-up study of sonelokimab to collect longer-term safety and efficacy data.
It is also evaluating sonelokimab in the Phase II ARGO trial for treating active psoriatic arthritis. The primary end-point readout from this study is anticipated in the fourth quarter of this year.





