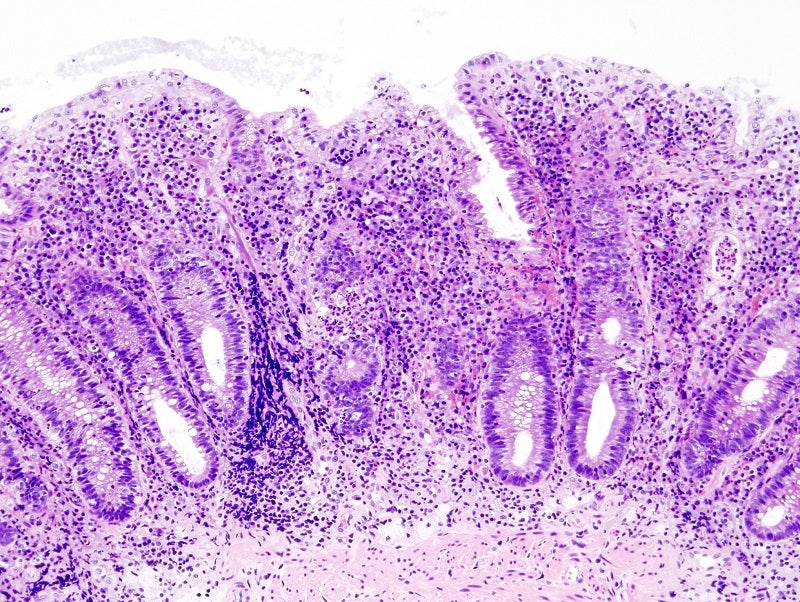
Biopharmaceutical company Morphic Therapeutic has dosed the first participant in the EMERALD-2 Phase IIb study of MORF-057 to treat moderate-to-severe ulcerative colitis (UC) patients.
The placebo-controlled, double-blind, randomised global Phase IIb study has been designed to evaluate the efficacy and safety of three active dose regimens of MORF-057 in adult UC patients.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The participants in the study will be randomised to receive either 200mg BID (twice daily) MORF-057, 100mg BID MORF-057, a QD (once daily) dose of MORF-057, or a placebo dose.
All the participants will receive MORF-057 for 40 weeks of maintenance dosing after the 12-week induction phase.
Clinical remission rate as measured by the Modified Mayo Clinic Score (mMCS) at 12 weeks will be the trial’s primary endpoint.
In the EMERALD-2 study, many secondary and exploratory endpoints will also be measured based on the mMCS, along with histologic, pharmacokinetic, and pharmacodynamic measures, and safety parameters.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMorphic Therapeutic CEO Praveen Tipirneni said: “MORF-057 offers the possibility of efficacy and tolerability through the proven mechanism of α4β7 inhibition, together with the ease and convenience of oral dosing for patients suffering from IBD.
“We are therefore extremely excited to begin the EMERALD-2 study of MORF-057. In addition, we are now eagerly looking forward to the results of the EMERALD-1 Phase IIa study of MORF-057 that we expect in the second quarter of 2023.”
An oral small molecule α4β7 integrin inhibitor, MORF-057 is being developed for patients with inflammatory bowel disease (IBD).
Similar to vedolizumab, the integrin inhibitor has been designed for blocking the interactions between α4β7 on the lymphocytes’ surface and the mucosal endothelial cell ligand MAdCAM-1.
This will reduce the lymphocyte migration from the bloodstream to intestinal mucosal tissues.
In addition, the company expects to receive top line results from EMERALD-2 in the first half of 2025.



