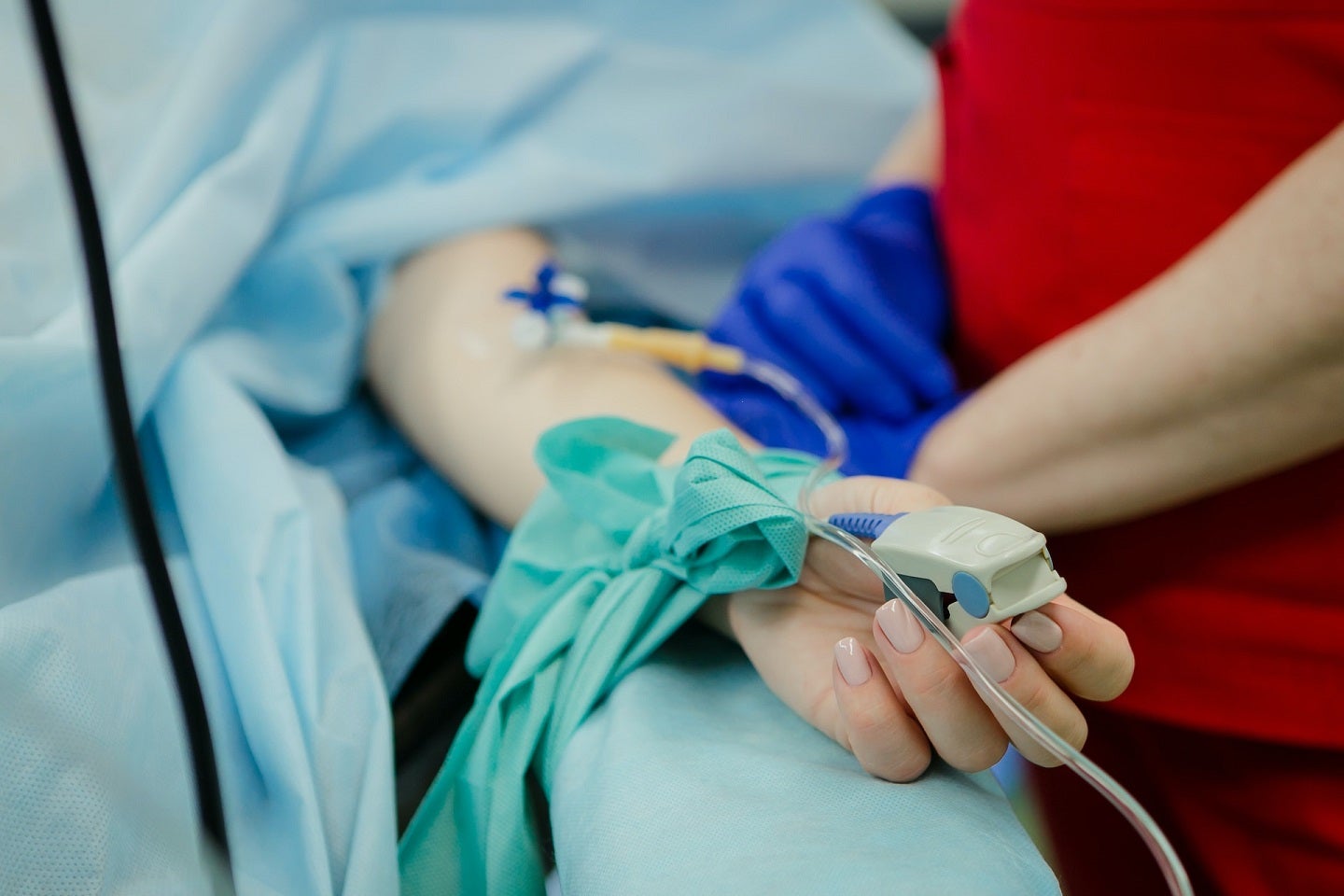
Mythic Therapeutics has dosed the first subject in the Phase l KisMET-01 trial of MYTX-011 to treat locally advanced, recurrent or metastatic non-small cell lung cancer (NSCLC) patients.
The patient was dosed at Sarah Cannon Research Institute (SCRI) at Tennessee Oncology in Nashville, US, under the supervision of SCRI Lung Cancer Research director Melissa Johnson.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
MYTX-011 is an investigational cMET targeting ADC that features Mythic’s FateControl technology, which has the potential to improve ADC efficacy.
The open label, multi-centre, dose escalation and dose expansion KisMET-01 trial aims to assess the safety, tolerability, pharmacokinetics and preliminary anti-tumour activity of MYTX-011 in locally advanced, recurrent or metastatic NSCLC patients.
It will feature two parts, with the first part evaluating the safety and tolerability of MYTX-011 and identifying the dose(s) of the drug to be studied in the next part.
The second part will have patients with NSCLC with cMET overexpression or MET amplification/exon 14 skipping mutations.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMythic Therapeutics chief scientific officer and co-founder Brian Fiske said: “Today’s announcement of our first subject dosed represents a significant step towards increasing the number of lung cancer patients eligible for treatment using ADCs, including those whose cancers express moderate cMET levels.
“At Mythic, we are focused on our vision of unlocking the full potential of MYTX-011 as well as our broader pipeline of ADCs incorporating FateControl technology, which represents a fundamentally new paradigm for ADC therapies.”





