Neurimmune has reported primary results from the Phase I clinical trial of NI006, a human monoclonal antibody designed for the depletion of amyloid deposits in Amyloid transthyretin cardiomyopathy (ATTR-CM).
An underdiagnosed, systemic condition, ATTR-CM leads to heart failure and may cause death within four years from diagnosis.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The proof-of-concept, first-in-human study has randomised patients to receive ascending doses of NI006 for 12 months and determine its safety, efficacy, pharmacology, and tolerability.
Results demonstrated the safety profile of NI006 up to the highest dose levels tested.
No drug-related serious adverse reactions and antidrug antibodies were detected during the study. Pharmacokinetic profile was also seen to be consistent with that of an IgG antibody.
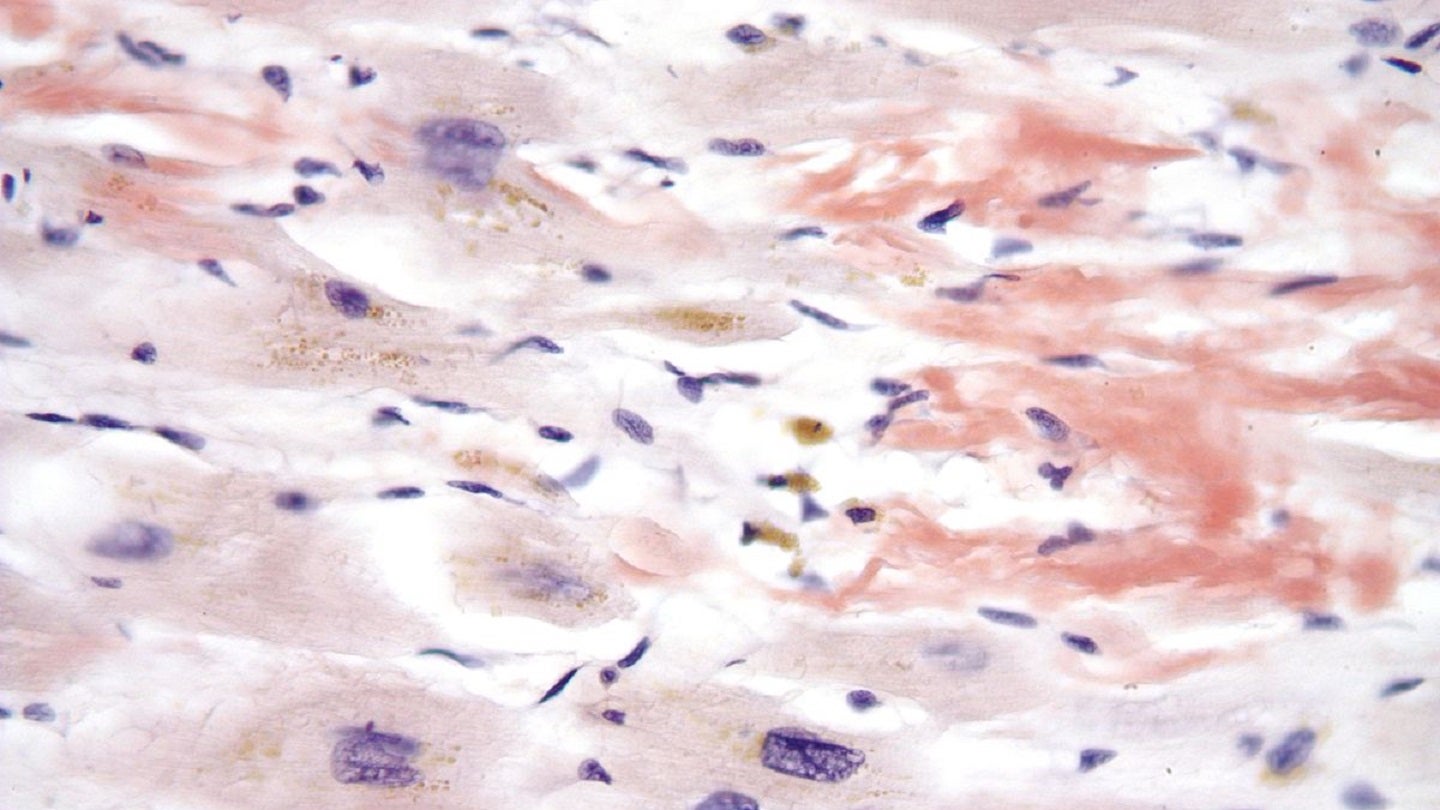
Cardiac amyloid deposition, measured using scintigraphy or cardiac magnetic resonance imaging, was reduced at a dose of at least 10mg per kilogram given to participants over the 12 months of treatment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLevels of biomarkers evaluating N-terminal pro-B-type natriuretic peptide and troponin T, cardiac stress and cardiomyocyte death were also reduced in patients receiving NI006.
Last January, AstraZeneca’s Rare Disease group Alexion and Neurimmune signed an exclusive worldwide partnership and licence agreement to develop NI006.
Neurimmune will oversee the completion of the Phase Ib trial on behalf of Alexion, which will provide trial expenses.
Neurimmune chief medical officer Christoph Hock said: “We thank all patients, their families and investigators, study staff and collaborators for the participation in the NI006 first-in-human study.
“The NI006 results warrant further development of this drug candidate with the potential of reverting disease progression in ATTR amyloidosis.”
As per the deal, Alexion will receive an exclusive global licence for the development, production and marketing of NI006.



