AVEO Oncology and Astellas have discontinued BATON (Biomarker Assessment of Tivozanib in ONcology), the breast cancer clinical trial of its lead drug ‘tivozanib’ due to insufficient patient enrolment.
The Phase II trial is intended for patients with locally recurrent or metastatic triple negative breast cancer (TNBC).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company was developing the drug in collaboration with Astellas Pharma. It said enrolment in the trial had been slower than expected and did not improve despite efforts to recruit more patients.
AVEO chief medical officer William Slichenmyer said while the company believes in the potential benefits of tivozanib for patients with triple negative breast cancer, it has decided to discontinue the trial because of low patient accrual.
"We want to thank the study investigators and their patients who participated in the trial for their support," Slichenmyer said.
Patient enrolment in the randomised, double-blind, multicentre Phase II clinical trial started in December 2012.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe BATON-BC trial was testing the efficacy of tivozanib in combination with paclitaxel compared with placebo in combination with paclitaxel in patients suffering locally recurrent or metastatic triple negative breast cancer, who have received no more than one systemic therapy for advanced or metastatic breast cancer.
AVEO and Astellas have equally shared all committed expenses related to the BATON-BC trial.
In addition, data from a planned interim analysis of the Phase II trial of tivozanib in patients with colorectal cancer indicated that the trial was unlikely to meet the primary endpoint in the intent-to-treat patient population.
Interim data is being assessed, and AVEO and Astellas are currently in discussions regarding future steps involving the drug.
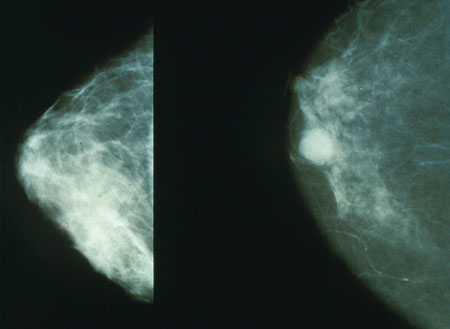
Image: Mammograms showing a normal breast (left) and a cancerous breast (right). Image: courtesy of Morning2k.





