
Israeli biotechnology firm Can-Fite BioPharma is set to commence patient enrolment for a Phase II clinical trial of namodenoson to treat non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), following the successful conclusion of a clinical investigator meeting.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Namodenoson is a small orally bioavailable drug with a high affinity to selectively bind to the A3 adenosine receptor (A3AR) and ability to differentiate normal cells from diseased ones.
The multi-centre, randomised, double-blinded, placebo-controlled, dose-finding Phase II trial will evaluate the efficacy and safety of the drug in 60 patients with NAFLD and with or without NASH.
During the treatment period, two different dosages of oral, twice a day namodenoson will be compared to placebo.
Can-Fite BioPharma medical director Dr Michael Silverman said: “We conducted a successful Investigator Meeting with clinical researchers eager to evaluate namodenoson in the treatment of NAFLD / NASH.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Details of the Phase II clinical trial protocol were reviewed and questions and comments from clinicians were addressed.
“We are pleased to have some of the most prestigious medical institutions, including Hadassah Medical Centre and Rabin Medical Centre serve as clinical sites for the trial.”
The trial is based on namodenoson’s pre-clinical studies that demonstrated the drug’s efficacy to mitigate liver fat in NASH, improve liver function and regenerate liver cells.
The primary endpoints of the Phase II trial include safety and percentage change in liver triglyceride (fat) concentration from baseline as measured by nuclear magnetic resonance spectroscopy (NMRS).
Can-Fite is also studying namodenoson as a second line treatment in another Phase II trial for hepatocellular carcinoma.
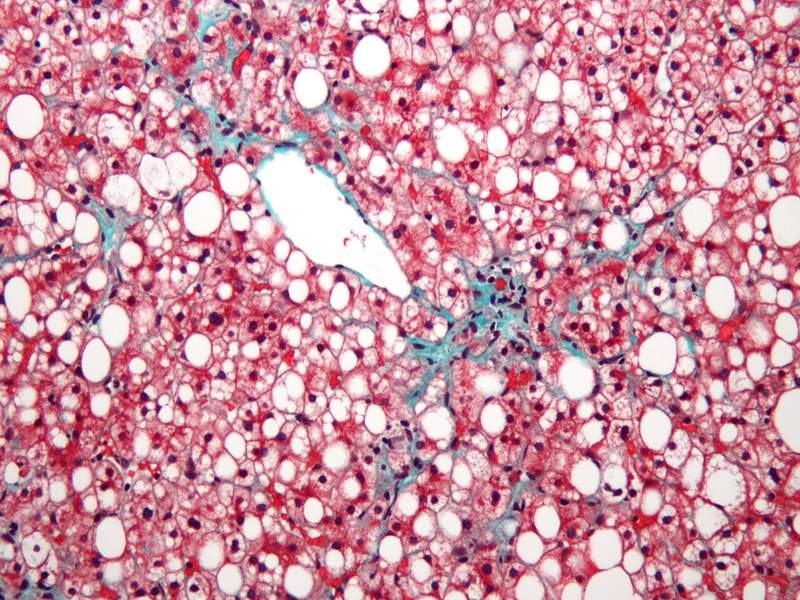
Image: Micrograph of non-alcoholic fatty liver disease (NAFLD). Photo: courtesy of Nephron.





