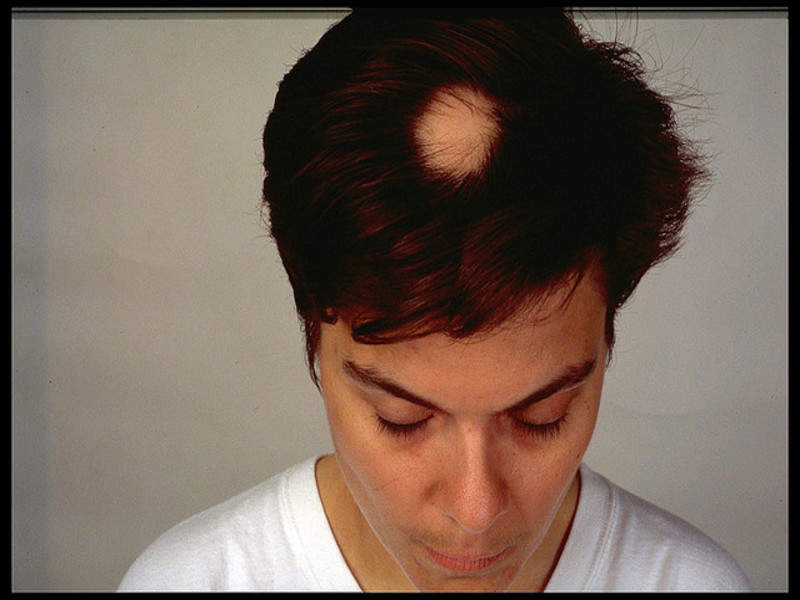

US-based Concert Pharmaceuticals has commenced the Phase IIa clinical trial of CTP-543 to treat patients with moderate-to-severe alopecia areata.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
CTP-543 was discovered using the firm's deuterium chemistry technology and is an orally administered, selective Janus kinase 1 (JAK 1) and Janus kinase 2 (JAK 2) inhibitor.
The double-blind, randomised, placebo-controlled, parallel-dose Phase IIa trial will assess the safety and efficacy of CTP-543 in up to 100 patients following 12 months of treatment.
The trial will compare twice-daily 4mg, 8mg, 12mg and 16mg doses of CTP-543 with placebo.
Concert Pharmaceuticals president and chief executive officer Roger Tung said: “Alopecia areata is a devastating autoimmune disease that impacts half a million or more patients in the US at any given time.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“We intend to be at the forefront of advancing a new oral treatment for alopecia areata patients and look forward to assessing CTP-543’s therapeutic efficacy in the Phase II trial.”
During the trial, the primary analysis will be performed at week 24 and conducted using the severity of alopecia tool (SALT).
An additional CTP-543 dosing for a period of 28 weeks will also be included for all the participating patients.
The top-line results from the trial are expected to be reported in the first quarter of next year.
The results from Phase I trials of CTP-543 conducted in healthy volunteers showed that the product was well tolerated without serious adverse events and demonstrated enhanced exposure with increasing doses.
Image: Patient suffering from patchy hair loss due to alopecia areata. Photo: courtesy of Carolyn P Speranza.





