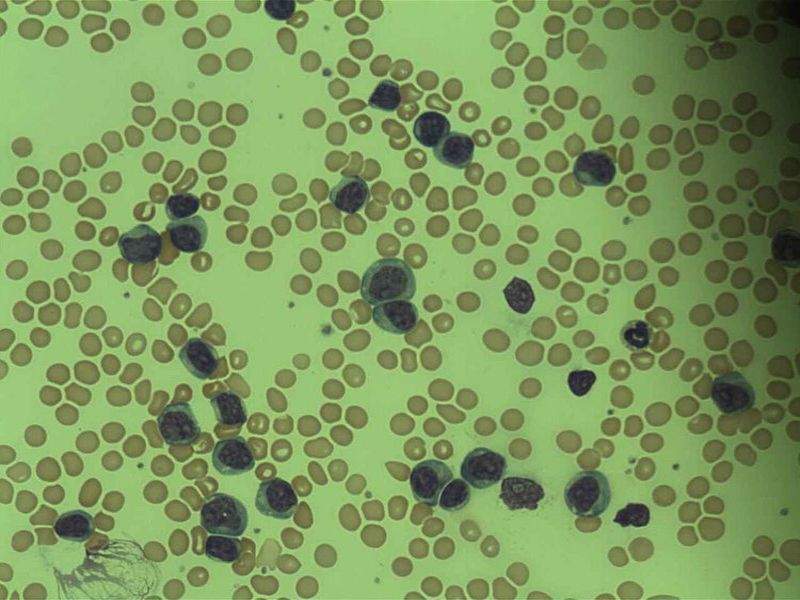

French biopharmaceutical firm Erytech Pharma has begun a Phase II clinical trial of eryaspase (GRASPA) for the treatment of patients with acute lymphoblastic leukaemia (ALL).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Eryaspase contains an enzyme called L-asparaginase, encapsulated inside donor-derived red blood cells using the firm's ERYCAPS platform.
The single arm, multi-centre, investigator-initiated, multi-national Phase II trial is designed to evaluate the combination of eryaspase and Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL 2008 multi-agent chemotherapy protocol in 30 subjects.
The trial is to be conducted in partnership with NOPHO and will recruit patients at 23 sites in seven Nordic and Baltic countries, including Denmark, Finland, Norway, Sweden, Iceland, Lithuania and Estonia.
Erytech Pharma chief medical officer Dr Iman El-Hariry said: "Collaborating with NOPHO is an exciting opportunity for Erytech to evaluate eryaspase in this specific patient population, with the potential to demonstrate expanded application of our technology to treat blood cancers where drug resistance and hypersensitivity clinical reactions to first-line chemotherapies is common.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData"This study aligns with our global strategy to develop the ERYCAPS technology with an increased tolerability and efficacy profile for patients who may respond to L-asparaginase when it is delivered through encapsulated red blood cells."
The combination therapy will be given as a second-intention treatment to children or adult patients with silent inactivation or hypersensitivity reactions to PEG-asparaginase.
Eryaspase is currently under assessment in a Phase IIb trial in Europe for elderly patients with acute myeloid leukaemia (AML) alongside a Phase II trial in metastatic pancreatic cancer patients, and also in a Phase I trial in the US to treat adults with relapsed or refractory ALL.
Image: Micrograph of acute lymphoblastic leukaemia. Photo: courtesy of Osaretin/Wikipedia.





