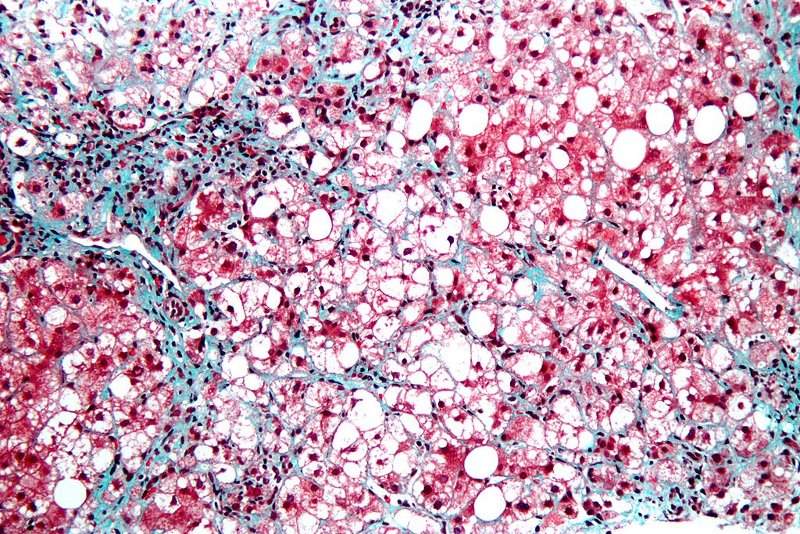

Israeli-based clinical-stage bio-pharmaceutical company Galmed has closed patient enrolment for its Phase IIb ARREST study of Aramchol to treat liver diseases such as non-alcoholic steato-hepatitis (NASH).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Orally administered aramchol is a conjugate of cholic acid and arachidic acid and belongs to synthetic Fatty-Acid / Bile-Acid Conjugates (FABACs).
It partially obstructs activity of Stearoyl Coenzyme A Desaturase 1 (SCD1) in the liver, resulting in a reduced synthesis of fatty acids with a subsequent decrease in storage of triglycerides and other esters of fatty acids, thereby reducing liver fat.
The Phase IIb ARREST study will be conducted as a global, multi-centre, randomised, double blind, placebo-controlled trial, which will test the safety and efficacy of Aramchol while examining 240 patients with NASH who are overweight or obese, and who are pre-diabetic or type-II-diabetic.
The study is primarily focused on achieving reduction in liver fat content measured by magnetic resonance spectroscopy (MRS).

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial will also explore its secondary histological endpoints of achieving improvement in fibrosis, two-point improvement in NAS (NAFLD Activity Score) and resolution of NASH, as well as assess surrogate metabolic endpoints.
ARREST study global principal investigator Professor Vlad Ratziu said: "The ARREST study is the largest study to date to incorporate both MRI and histology endpoints.
“I expect that Aramchol will demonstrate the effects seen in pre-clinical and clinical studies on the three pathologies of NASH: steatosis, inflammation and fibrosis.”
The study will be conducted for a period of 52 weeks followed up with a 12 weeks phase, with its result expected to be released in the second quarter of 2018.
Image: Micrograph displaying steatohepatitis. Photo: courtesy of Nephron.





