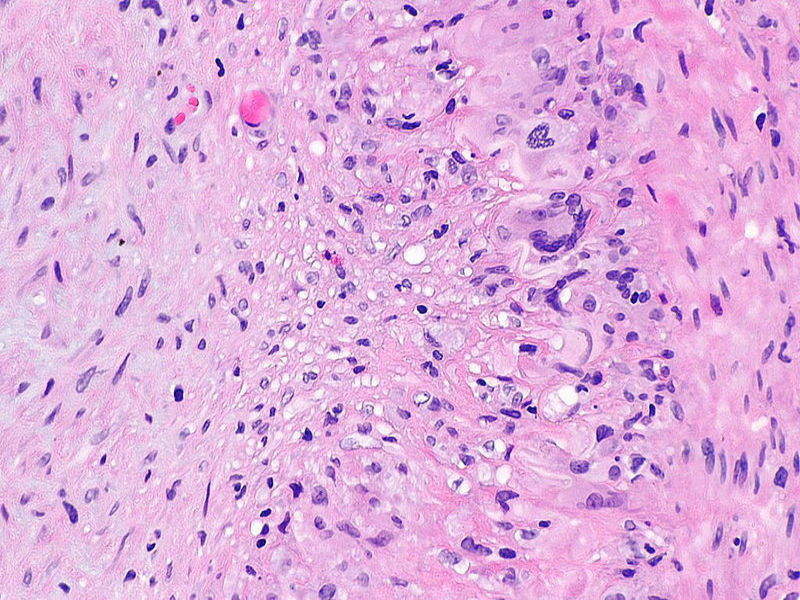

Genentech has reported positive results from the Phase III GiACTA clinical trial of Actemra (tocilizumab) to treat adults with giant cell arteritis (GCA).
Previously approved to treat active rheumatoid arthritis (RA) in adults, Actemra is an antagonist of the humanised interleukin-6 (IL-6) receptor.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
GiACTA is a global, randomised, multi-centre, double-blind, placebo-controlled Phase III trial. It evaluated the efficacy and safety of subcutaneous Actemra in 251 subjects at 76 clinical centres in 14 countries.
Results showed that the trial met its primary and secondary endpoints with 53.1% of sustained remission at 52 weeks with biweekly Actemra and 56% when given weekly Actemra in combination with a 26-week steroid taper regimen.
In case of patients who were administered a combination of placebo and a 26-week steroid taper regimen, sustained remission was found to be 14%.
The US Food and Drug Administration (FDA) approved subcutaneous Actemra for GCA treatment in May this year.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataGenentech chief medical officer and global product development head Sandra Horning said: “The data from GiACTA supported the FDA approval of the first new treatment option for patients with GCA in more than 50 years, and we are committed to continuing to explore new treatment options for autoimmune diseases with significant unmet medical need.”
During the GiACTA trial, the safety profile observed for Actemra was found to be consistent with its documented safety profile in other indications.
Actemra is studied as an intravenous administration in five Phase III trials with 4,000 RA patients in 41 countries and in two clinical trials as subcutaneous infusion for 1,800 RA subjects in 33 countries.
Image: Micrograph of giant cell arteritis. Photo: courtesy of Nephron.





