GlaxoSmithKline (GSK) has started dosing in a Phase III trial evaluating sirukumab, a human anti-interleukin (IL)-6 monoclonal antibody, to treat patients with giant cell arteritis (GCA).
Sirukumab is an investigational human anti-IL-6 monoclonal antibody. It binds with high affinity to the IL-6 cytokine, a naturally occurring protein believed to play a role in autoimmune conditions.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Sirukumab is currently in phase III development for rheumatoid arthritis (RA) and GCA.
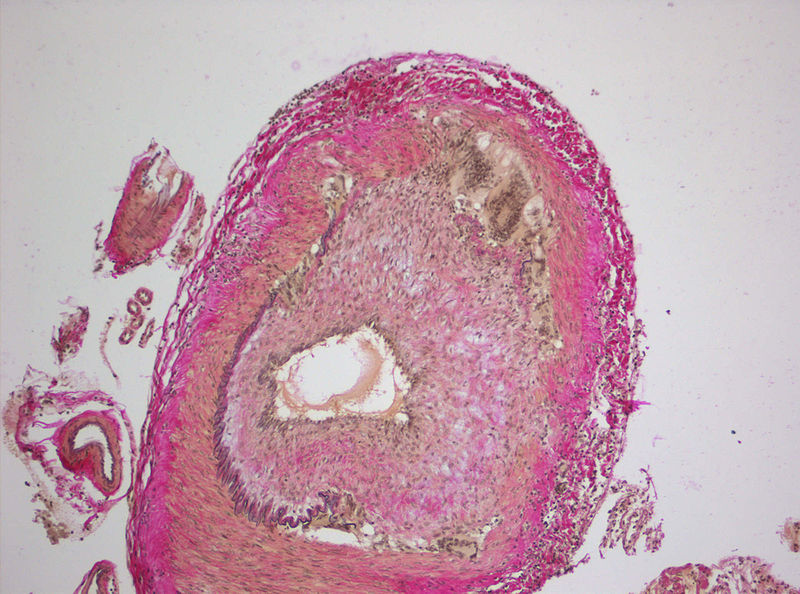
Occuring in people aged 50 years or over, the latter disease is characterised by inflammation of large and medium-sized arteries predominantly in the head and neck.
GCA patients usually experience severe headache, sight loss, jaw and muscular pain. If the disease is not treated promptly, they may be at risk of permanent sight loss.
The Phase III trial (SIRRESTA) is designed to assess the efficacy and safety of two subcutaneous doses of sirukumab 100mg every two weeks, and 50mg every four weeks, with a pre-specified tapering dose of prednisone to treat GCA.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to GSK, this approach will test whether treatment with sirukumab can reduce the duration of steroid treatment typical in clinical practice.
The trial includes two distinct parts: a 52-week double-blind treatment phase (part A), and a 104-week extension phase (part B). Its primary endpoint is the proportion of patients that achieve sustained remission.
GSK Immuno-Inflammation R&D senior vice-president Paul-Peter Tak said: "The use of high dose steroids to treat giant cell arteritis can cause severe side effects with prolonged use.
"Alternative treatments are required and we believe sirukumab could be an important option for patients with this disease.
"The start of our study with sirukumab for GCA, which is currently under investigation for rheumatoid arthritis, marks the progress we are making to apply our knowledge of the underlying cause of a variety of immune-mediated inflammatory diseases, and explore the potential of our immuno-inflammation pipeline to treat multiple conditions."
GSK noted that sirukumab is not approved as a treatment for any indication anywhere in the world.
As part of a collaboration with Ireland-based Janssen Biologics, the company initiated a Phase III programme in August 2012 to evaluate sirukumab as a treatment for moderately to severe RA.
Signed by the two companies in December 2011, a licensing and co-development collaboration agreement allows them to investigate sirukumab for other indications beyond RA.
Image: Histopathology of giant cell vasculitis in a cerebral artery. Photo: courtesy of Marvin 101.





