
US-based biotechnology company Imara has initiated its Phase I clinical study of IMR-687 to treat patients with sickle-cell disease and other haemoglobinopathies.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
IMR-687 is an orally administered, highly potent candidate developed to selectively inhibit phosphodiesterase-9 (PDE9i) in blood cells.
The inhibition of PDE9 reduces white blood cell stickiness that prevents blockage of blood vessels.
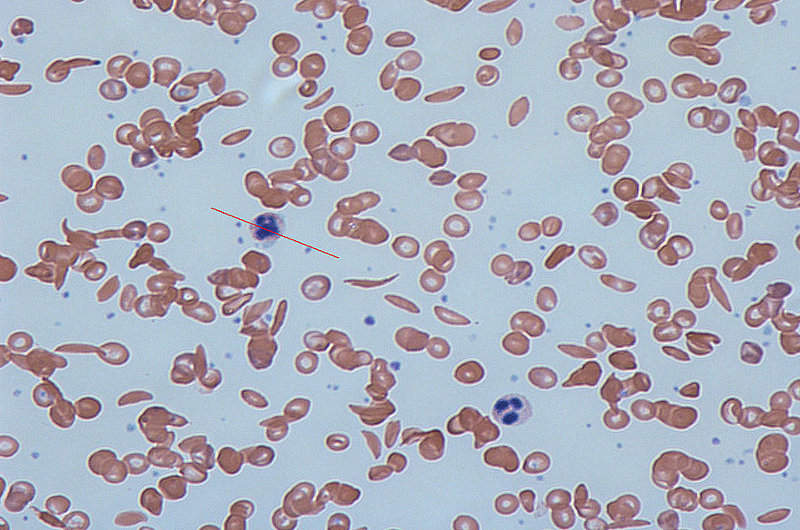
Sickle-cell disease is a rarely occurring, genetically inherited condition that alters haemoglobin, which is responsible for transporting oxygen throughout the body.
Altered haemoglobin distorts red blood cells into a stiff and inflexible sickle, or crescent, which gets stuck in small blood vessels.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe condition prevents blood carrying oxygen from reaching tissues and organs, thereby leading to vaso-occlusive crisis (VOC), acute chest syndrome (ACS), and permanent damage to organs including the liver, spleen, kidney and brain.
The Phase I clinical study is being conducted to assess the safety and tolerability of Imara’s lead product candidate, IMR-687.
Imara founder, president and CEO James McArthur said: “More than 160,000 individuals are living with sickle-cell disease in the United States and Europe with many more in Africa and Asia, yet, there remains a serious medical need facing those whose lives are burdened by this devastating disease.
“Initiating this first study in humans is a critically important milestone as we work to advance new therapies for people living with sickle-cell disease.”
Preclinical studies have suggested the efficacy of IMR-687 in reducing both the sickling of red blood cells and blood vessel occlusion.
Image: Human blood with normal and sickle-shaped cells. Photo: courtesy of Dr Graham Beards.





