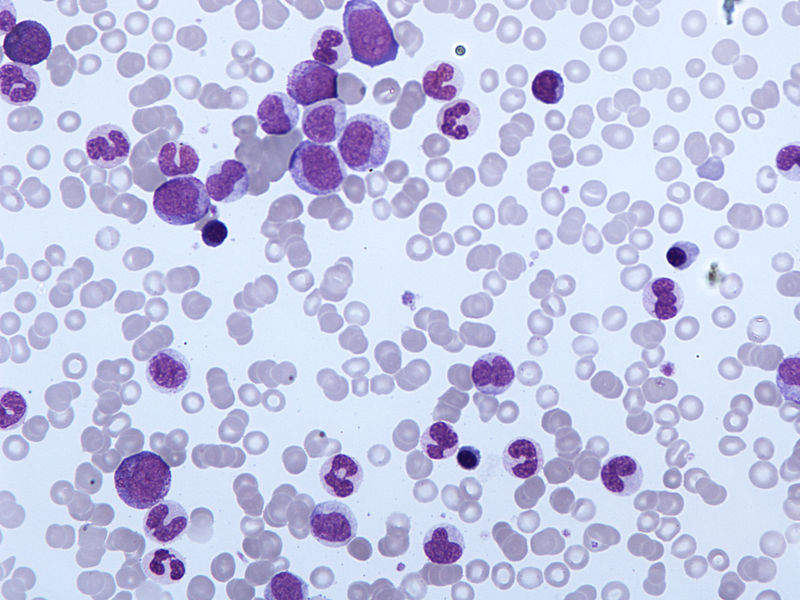
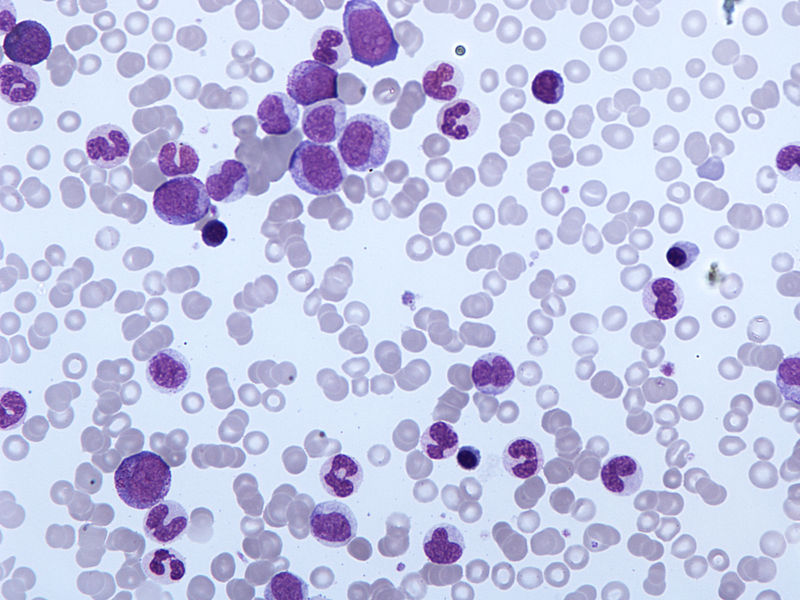
Immune Pharmaceuticals has started patient recruitment for Phase I/II clinical trial of Ceplene (histamine dihydrochloride) to treat chronic myelomonocytic leukaemia (CMML) patients.
Ceplene is an immunostimulant administered in combination with low-dose interleukin-2 (IL-2) to maintain the first remission in patients with acute myeloid leukaemia (AML).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The drug candidate is said to trigger immune-mediated destruction of leukaemic cells by working against dysfunction and apoptosis of T and NK cells. The open-label, non-randomised, multi-centre Phase I/II trial will enroll a total of 15 adult patients.
Funded by Sweden’s University of Gothenburg, the trial will investigate the safety and efficacy of combination of Ceplene and low-dose IL-2, Proleukin.
University of Gothenburg Institute of Biomedicine professor Kristoffer Hellstrand said: "CMML cells exert immunosuppression that is targeted by Ceplene.
"By reducing immunosuppression, Ceplene improves the function of anti-leukaemic immune cells, including T cells and natural killer (NK) cells."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataImmune's immuno-oncology unit Cytovia will execute the development and commercialisation plan for Ceplene and will offer a non-restricted grant to the University of Gothenburg to cover IL-2 treatment costs.
An international Phase III trial of Ceplene demonstrated the ability of the histamine dihydrochloride to prevent relapse of leukaemia in AML patients in the first remission and maintain quality of life during the treatment period.
The safety and efficacy results from the Phase III trial were further validated by a Phase IV trial.
The combination of Ceplene and low-dose Proleukin are approved in more than 30 European countries and in Israel to treat AML for maintenance of remission and prevention of leukaemia relapse.
Image: Peripheral blood film of chronic myelomonocytic leukaemia. Photo: courtesy of Simon Caulton.





