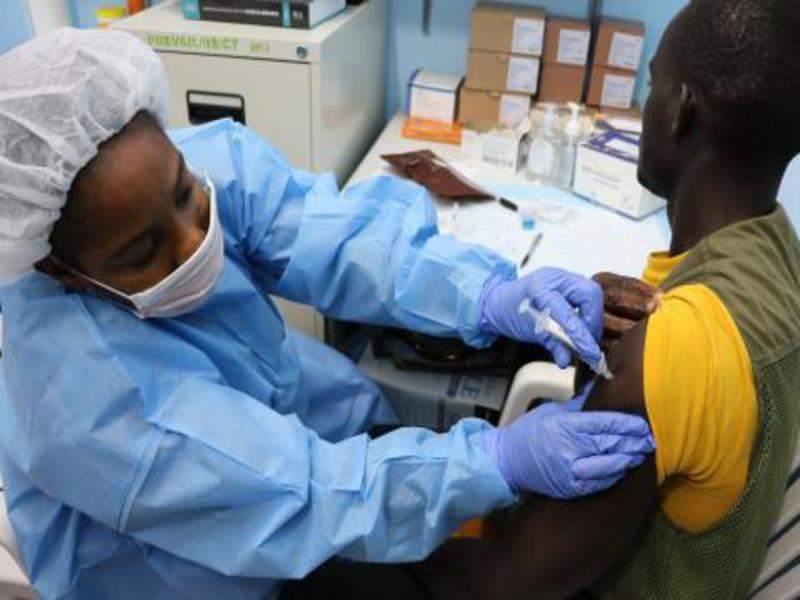

A new Phase II clinical trial has begun in West Africa to evaluate three vaccination strategies for the prevention of Ebola virus.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The French National Institute of Health and Medical Research (Inserm), the US National Institutes of Health (NIH) and the London School of Hygiene & Tropical Medicine (LSHTM) are conducting the trial under the Partnership for Research on Ebola Vaccination (PREVAC) international consortium.
The vaccine candidates for the PREVAC trial are being supplied by pharmaceutical firms Janssen Vaccines & Prevention, Bavarian Nordic and Merck Sharp & Dohme.
The two-stage, randomised Phase II trial will compare the three vaccination strategies with placebo regimens in more than 5,000 adults and children to determine the most potential strategy.
The rapidity, intensity and duration of the immune responses, as well as the safety and tolerability of the vaccines will also be examined.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe first stage of the trial is to be performed in Guinea and Liberia and will assess the combination of Janssen and Nordic experimental vaccines in 600 subjects aged 12 years and above.
A first dose of Ad26.ZEBOV will be followed by a booster dose of MVA-BN-Filo after eight weeks.
Expected to be initiated in the second half of this year, the second stage of the trial will evaluate the first stage regimen, alongside two other regimens, rVSVG-ZEBOV-GP4 and rVSVG-ZEBOV-GP, which include the vaccine by Merck.
This part of the trial will include 3,500 healthy participants aged 18 or older and 1,400 children aged one to 17.
The firm intends to further conduct the trial at Sierra Leone, in addition to the sites in Guinea and Liberia.
Image: Study volunteer receiving inoculation. Photo: courtesy of National Institute of Allergy and Infectious Diseases (NIAID).





