US-based biotechnology company OncoSec has entered a clinical trial collaboration and supply agreement with Merck to begin the Phase II PISCES clinical trial of ImmunoPulse IL-12 and keytruda combination for the treatment of patients with metastatic melanoma.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Keytruda (pembrolizumab) is Merck’s anti-PD-1 therapy, while OncoSec’s ImmunoPulse is an investigational technology developed to increase the local delivery and uptake of DNA-based immune-targeting agents such as IL-12.
Under the agreement, OncoSec will sponsor and fund the study, while Merck will provide keytruda.
The trial will assess the safety and efficacy of the combination in stage III/IV metastatic melanoma patients who are experiencing disease progression after prior treatment with an anti-PD-1 therapy.
OncoSec president and chief executive officer Punit Dhillon said: “This collaboration is supported by our recent clinical data demonstrating the potential ability of ImmunoPulse IL-12 to rescue patients who do not initially respond to anti-PD-1 therapy in melanoma.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“In addition to our recent fast-track designation for this population, OncoSec is uniquely positioned to meaningfully impact clinical outcomes for patients who do not currently have any other options.
“By working with innovative immuno-oncology leaders, this alliance underpins OncoSec's strategy to combine our ImmunoPulse IL-12 programme with checkpoint inhibitor therapies to advance the care of patients."
ImmunoPulse IL-12 has reportedly shown anti-tumour activity and a favourable safety profile in Phase I and II trials for the treatment of different solid tumours.
OncoSec is further assessing ImmunoPulse IL-12 in patients with triple-negative breast cancer.
The firm is also working towards developing new immune-targeting agents for use with their ImmunoPulse.
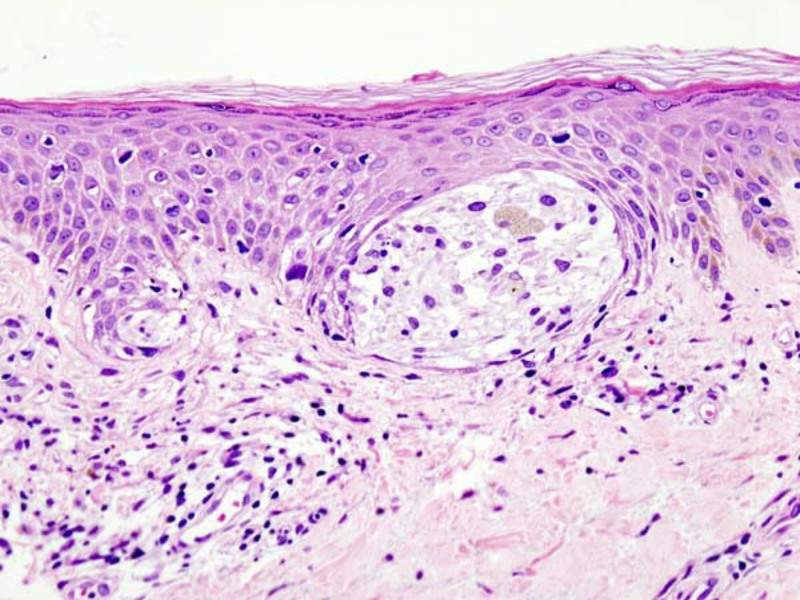
Image: Histopathologic image of malignant melanoma. Photo: courtesy of KGH via Wikipedia.





