Otonomy has started patient enrolment in the US Phase III clinical trial of OTO-104, a sustained-exposure formulation of the steroid dexamethasone, to treat Ménière’s disease.
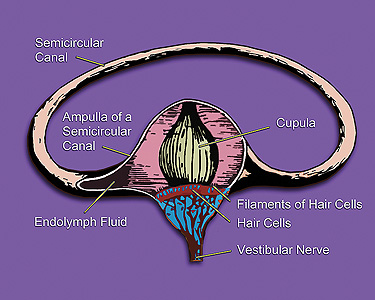
Also called endolymphatic hydrops, Menière’s disease is a disorder of the inner ear that can affect hearing and balance. It is characterised by acute vertigo attacks, tinnitus, fluctuating hearing loss, and a feeling of aural fullness.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The company noted that a second Phase III trial is expected to be initiated in the European Union (EU) in early 2016.
Around 160 patients with unilateral Ménière’s disease will be enroled in the 16-week Phase III trial at multiple US clinics.
Otonomy president and CEO David Weber said: "Initiation of Phase III for OTO-104 in Ménière’s disease marks an important milestone for this programme and for our company, whose founder suffers from this debilitating vertigo disorder.
"We look forward to leveraging the valuable lessons we learned from our Phase IIb trial, and reviewed during our End-of-Phase II meeting with the FDA, to successfully implement this Phase 3 program with a goal of providing results in the second half of 2017."

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial’s primary endpoint is efficacy of OTO-104, which will be measured by the reduction in number of definitive vertigo days (DVD) in the third month of the trial.
OTO-104 has also been granted fast-track designation by the US Food and Drug Administration (FDA).
The company has completed a Phase IIb trial evaluating OTO-104 in 154 patients with unilateral Ménière’s disease. Top-line results showed that OTO-104 narrowly missed the primary efficacy endpoint.
The data showed that OTO-104 achieved statistical significance for multiple secondary vertigo endpoints at different time points.
Image: An illustration of one of the three semicircular canals of one inner ear and associated structures. Photo: courtesy of DavidMaisel.





