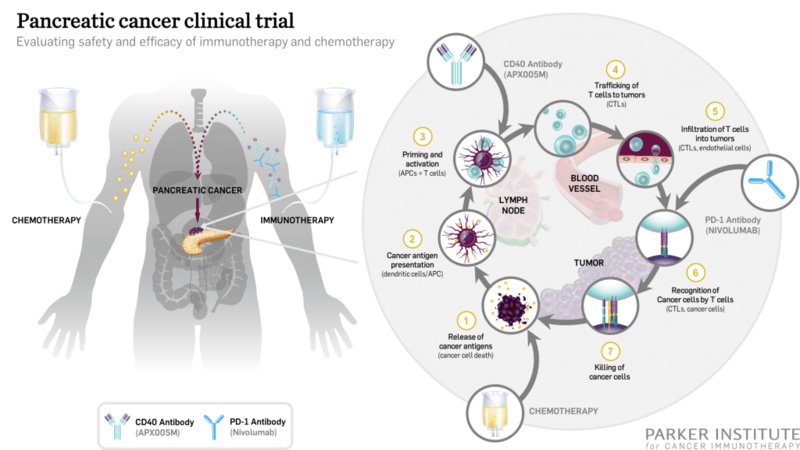
The Parker Institute for Cancer Immunotherapy and Cancer Research Institute in the US have started dosing patients in a Phase Ib/II clinical trial to investigate the combination of standard chemotherapy and immunotherapy for the treatment of pancreatic cancer.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The institutes have partnered with Bristol-Myers Squibb (BMS) and biotech firm Apexigen for the supply of investigational immunotherapy agents for the trial.
BMS’ anti-PD-1 checkpoint inhibitor nivolumab and Apexigen’s new monoclonal antibody that targets CD40 protein, APX005M, will be evaluated with chemotherapy.
Led by the University of Pennsylvania’s Abramson Cancer Centre, the trial will be carried out at the Parker Institute’s network of cancer research centres.
Parker Institute for Cancer Immunotherapy clinical development vice-president Ramy Ibrahim said: “A trial of this magnitude has many moving parts: multiple contracts and co-funders, a four-drug IND, a major industry partner, a biotech firm, and six university hospitals.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Leveraging the Parker Institute’s infrastructure, we streamlined the process and successfully launched the trial in less than six months, perhaps half the time it would have taken otherwise.”
The open-label, multi-centre Phase Ib/II trial will assess the safety and efficacy of the combination therapies in patients with previously untreated metastatic pancreatic adenocarcinoma.
During the trial, two standard-of-care chemotherapy drugs, gemcitabine and nab-paclitaxel, will be combined with APX005M, with or without nivolumab.
The primary outcome measure of the Phase Ib part is proportion of patients with adverse events (AEs), serious adverse events (SAEs) and dose-limiting toxicities, and overall survival in the Phase II part.
Estimated to recruit approximately 105 subjects, the trial is due to be completed in 2022.
Image: Illustration of the Phase Ib/II pancreatic cancer trial of chemotherapy and immunotherapy combination. Photo: courtesy of The Parker Institute for Cancer Immunotherapy.





