
US-based biotechnology firm Peloton Therapeutics has started dosing patients in its Phase II clinical trial of PT2385 for the treatment of kidney cancer caused by von Hippel-Lindau (VHL) disease.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
PT2385 is an investigational, selective, orally active, small molecule drug designed to target hypoxia-inducible factor 2α (HIF-2α).
The tumour suppressor von Hippel-Lindau protein (pVHL) that acts on HIF-2α degradation is reportedly inactivated in clear cell renal cell carcinoma (ccRCC) tumours, resulting in the accumulation of HIF-2α and the transcription of genes, which leads to kidney cancer tumorigenesis.
The open-label Phase II trial is currently being performed in partnership with the National Cancer Institute (NCI) and will assess the efficacy, safety, pharmacokinetics and pharmacodynamics of the drug in patients with a minimum of one measurable VHL disease-associated ccRCC tumour.
Peloton chief executive officer John Josey said: “There is a significant need for new treatment options for VHL disease, a rare disease with serious and lifelong consequences for patients, and for which there are no approved systemic therapies.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The current standard of care for patients with VHL disease-associated kidney cancer is surgery, which commonly does not result in a cure for these patients.”
The administration of oral PT2385 will be continuous during the treatment period, except in the case of disease progression.
The primary objective of the trial is an examination of the overall response rate (ORR) of VHL-associated ccRCC tumours in untreated VHL patient.
The trial will also evaluate the changes in VHL-associated non-ccRCC lesions.
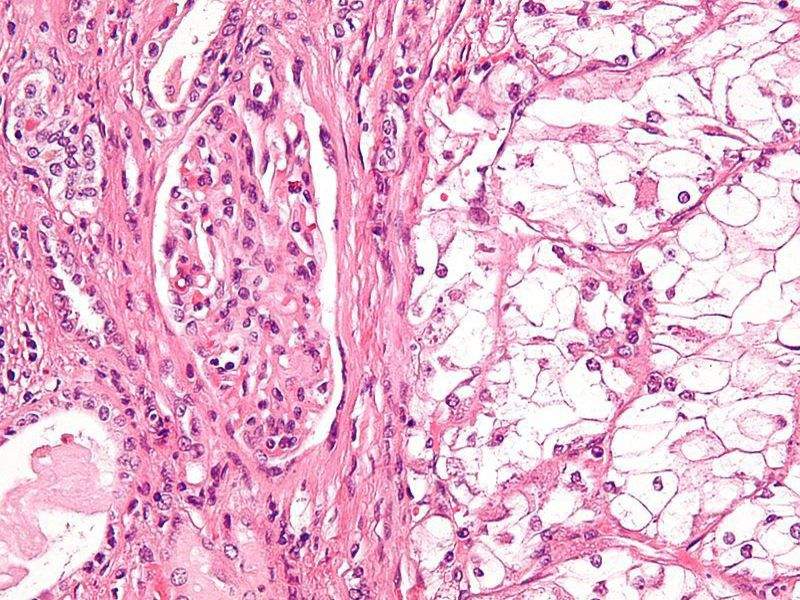
Image: Micrograph of a clear-cell renal cell carcinoma. Photo: courtesy of Nephron.





