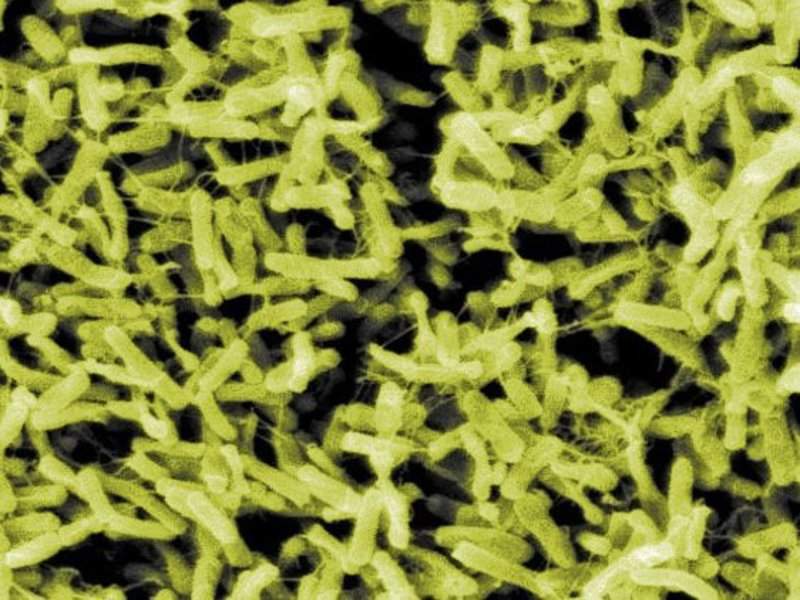

US-based clinical-stage microbiome firm Rebiotix has started patient enrolment in a Phase III clinical trial of RBX2660 to prevent recurrent Clostridium difficile (C. diff) infection.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
RBX2660 is developed using the firm’s Microbiota Restoration Therapy (MRT) platform, which is a stabilised drug technology that delivers spore and non-spore forming microbes into patient’s intestinal tract to restore healthier state of a dysbiotic gut.
The randomised, double-blind, multi-centre, placebo-controlled Phase III trial is designed to investigate the efficacy and safety of RBX2660 at clinical sites in the US and Canada.
While the trial’s primary endpoint is the proportion of subjects with treatment success after a blinded RBX2660 therapy, the treatment success will act as prevention of recurrent infection for eight weeks.
Rebiotix president and CEO Lee Jones said: “We plan to continue our strong momentum generated by our Phase II results in this Phase III trial as we seek to advance RBX2660 toward registration and potential approval so patients have an option for this unmet medical need.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Initiating the Phase III clinical study of RBX2660 is a significant milestone for Rebiotix and showcases the potential of our MRT platform to enable the development of microbiome-directed drug products.”
RBX2660’s Phase II programme for the prevention of C. diff infection included three separate Phase II trials, one of which was conducted as a randomised, double-blind, placebo controlled Phase IIb study.
The RBX2660 is reported to have been evaluated in up to 300 subjects to date, with a 24-month follow-up following treatment.
Image: Clostridium difficile bacteria. Photo: courtesy of Janice Carr/CDC.





